Odd resonance stabilisation observed in ground state opens door to studying poorly understood phenomenon
![Top view of the single-crystal X-ray diffraction structure of a dithienothiophene (DTT)-bridged [34]octaphyrin](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xany/3/6/2/132362_Bicyclic-Baird-type-aromaticity-fig-1-c.jpg)
The first compound that shows a peculiar form of resonance stabilisation known as bicycloaromaticity in its ground state has been made by an international team of researchers.
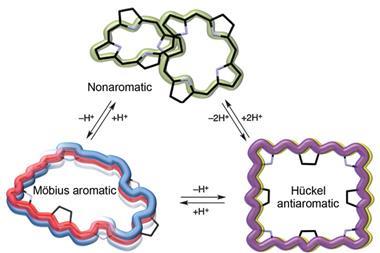
Bicycloaromaticity is similar to Möbius aromaticity – both occur in compounds with 4n π-electrons rather than the usual [4n+2] electrons found in Hückel-aromatic molecules like benzene. The former, however, appears only in triplet molecules, which, like oxygen, have two unpaired electrons. This rare form of π-electron stabilisation has only been observed a few times and always in short-lived high energy species.
![Oxidized forms display [4n ] π-electron aromatic character in accord with Baird’s rule](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/480xany/3/8/8/132388_nchem.2834-aop-Fig-4e.jpg)
The new compound, a large bridged porphyrin, is the first one that is bicycloaromatic in its ground state. Like most bicyclic molecules, it starts off with dual aromaticity – it has two competing π-electron systems that exist independently from one another. But after losing two electrons in an oxidation reaction and forming a biradical, the molecule’s aromatic systems merge, creating a single 40 electron π-system distributed over two rings.
While the compound doesn’t have any practical application yet, it gives scientists the opportunity to study a little understood form of aromaticity.
References
W-Y Cha et al, Nat. Chem., 2017, DOI: 10.1038/nchem.2834











No comments yet