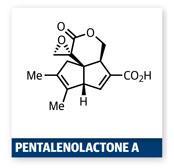
One team that really gets the PKR and all its nuances is that led by Zhen Yang at Peking University in Beijing, China. They recently published a very neat synthesis of the intricate pentalenolactone A methyl ester using Pauson–Khand technology to build the core 5,5-fused bicycle.2 Their goal was a worthwhile target – the family of pentalenolactones are important members of a group of antibiotics produced by prokaryotes.3 From the off, they were aware of the complications of using a Pauson–Khand reaction as the keystone to their synthesis, particularly with heavily substituted substrates.
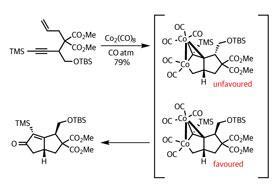
To mitigate this, the team spent some time evaluating potential substrates, and selected a suitable enyne starting point for the PKR (figure 1). The complex reaction with carbon monoxide generated their desired product as the correct stereoisomer in great yield, with the only downside being the lengthy reaction time – eight days! In this process, the team created two five-membered rings, two new stereocentres and installed key functional groups – so their diligence certainly paid off.
It wasn’t time to relax, though – their target required another two rings and a lot of decoration, so the team swiftly moved on, firstly methylating the enone, and secondly decarboxylating one of the methyl esters. They then used a remarkable domino reaction process developed by Richard Taylor to install a third ring, taking advantage of a small phosphonate sidechain (figure 2).4
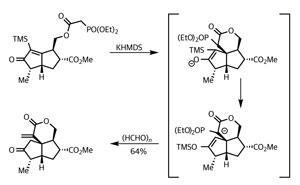
A little base deprotonated next to the phosphonate, promoting Michael addition into the enone. The resulting intermediate rearranged – the trimethylsilyl group migrating to the enol in a Brook rearrangement. This was then perfectly set for a Horner–Wadsworth–Emmons olefination with formaldehyde, returning their desired tricycle, and featuring the key exocyclic methylene. Phew!
With the two stars of their synthetic performance exiting stage left, the team was tantalisingly close to the natural product. But as we’ve often seen on this page, the functional group interconversions required to complete the route took some effort. Their first step was to methylate one of the alkenes via a modified Stille coupling of an enol triflate with the rather nasty tetramethyl stannane.
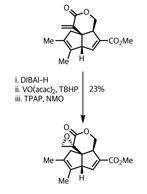
So the Pauson–Khand reaction yet again proves its worth. But I’ve still no idea how it works...
Paul Docherty is a science writer and blogger based in Reading, UK












No comments yet