Don’t let tired clichés get in the way of selective and sustainable chemistry
It seems impossible to talk about radicals without suggesting they should be tamed – and Chemistry World is no exception (in fact, we’re a routine offender). It’s a cliche as old as the hills, rooted in the notion that radicals are wild and uncontrollable. But it’s starting to look rather out of date. The truth is, chemists are well beyond taming many radicals; they’re putting them to work doing useful transformations in a mild, chemoselective and orthogonal manner.
In the middle of June, we reported how researchers in the US had created a microfluidic electrochemical flow cell that can control transient radicals just enough to do single-electron transfer radical–radical cross-coupling reactions. Normally, the radicals would be too unstable to do anything with, but using a device with tiny gaps between the anode and cathode means the speed at which the intermediates diffuse outpaces their decomposition. The result is a way to create C(sp2)–C(sp3) bonds without transition-metal catalysts.
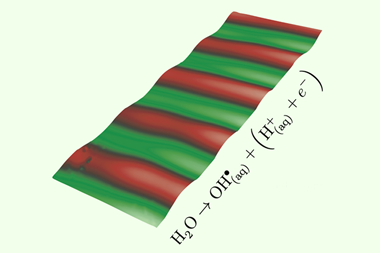
Less than a week later, we covered research detailing a unique mechanism for turning pure water into free radicals. The method shuns catalysts, electrolytes or electrodes. Instead, it uses vibrations from a piezoelectric material to trap and split water into hydroxyl radicals. What is unusual here is it doesn’t involve acoustic cavitation, which is typically associated with sonochemical reactions. No cavitation means no violent bubble implosions and the associated extreme temperatures and pressures. The team behind the work suggests the mechanism could be harnessed within a sustainable process for creating radicals that tackle water pollution by degrading organic pollutants.
And just like buses, a third radical story followed shortly after with a way to make the organic radical methyl viologen stable for up to six months in air. Changing its solvent from water to a deep eutectic solvent increases its lifetime around 100,000 times. Supramolecular shielding from the solvent environment protects the radical from oxygen while maintaining the electronic structure of the molecule. The team behind the work demonstrated the utility of the stabilised radical in an electrochromic device; the concept itself could be far wider reaching.
And let’s not forget that radical reactions are routine in polymer chemistry. Or that nature has been exploiting radical reactions in water for years.
It truly feels like radical chemistry has found momentum for making (and breaking) small molecules. So perhaps it’s time to talk differently about them, to better reflect the reality of modern chemistry. If we perpetuate past assumptions with lazy language, it risks dissuading new chemists from seeking out a powerful tool for their research. Let’s not stifle progress by using tired tropes to talk about a concept that could make a real difference to chemistry and society.









No comments yet