But those values are constantly refined
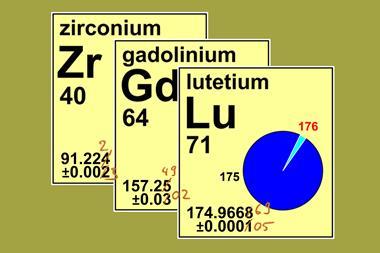
Last year, the International Union of Pure and Applied Chemistry (Iupac) revised the atomic weights of gadolinium, lutetium and zirconium. Gadolinium’s standard atomic weight changed from 157.250 to 157.249, lutetium’s went from 174.9668 to 174.96669, while zirconium’s went from 91.224 to 91.222. Those changes are minuscule, so why bother?
For most chemists, such adjustments will have little practical impact. But as Kit Chapman’s feature on atomic weights explains, at large or small scales, tiny differences are significant. That’s why the Iupac committee responsible for such updates scours the literature and meets regularly to deliberate which values should be reassessed.
When John Dalton set out his concept of atomic weights in the early 19th century, he proposed that each element consists of atoms with a definite weight and size. Today, we know that many elements can have natural isotopes, and the idea of a uniform atomic weight for an element’s atoms is not valid. Yet rather than diminishing the usefulness of atomic weights, understanding this variability has given scientists another tool for interrogating the world: using isotopic ratios to study environmental and biogeochemical processes and archaeology.
In the late 19th and early 20th centuries, the Harvard method – developed by Theodore Richards – used gravimetric measurements and chemical stoichiometry to determine atomic weights. This involved calculating the mass ratio of the element’s chloride or bromide to the chemically equivalent amount of silver or its corresponding silver halide. Richards would go on to win the 1914 Nobel prize in chemistry ‘in recognition of his accurate determinations of the atomic weight of a large number of chemical elements’ (although the Nobel committee only decided this in 1915).
In 1912, J J Thomson and Francis Aston observed that neon has two stable isotopes – neon-20 and neon-22 – explaining why the element’s measured atomic weight was 20.2. This finding led Aston to develop a higher-resolution mass analyser, ultimately confirming that elements can have multiple stable isotopes. As mass spectrometry and our knowledge of isotopes advanced, so too did the precision of atomic weight measurements.
For gadolinium, the 2024 update was its first since 1969. It was supported by high-precision measurements of the isotopic composition of gadolinium that used a regression model to circumvent an absence of gadolinium isotope standards. Indeed new experimental designs, alongside instrumental developments in mass spectrometry and technical improvements in separation science are the driving forces behind ongoing atomic weight updates.














No comments yet