Ben Valsler
Some time ago, @SimonBayly suggested on twitter that we look at nitrogenase – a class of enzymes that keep our ecosystems running smoothly. Here’s Katrina Krämer.
Katrina Krämer
In its pure form, nitrogen is a lazy element. As dinitrogen, it makes up almost 80% of our atmosphere’s volume. But unlike oxygen, which happily reacts with all sorts of things and is the gas of choice for breathing, nitrogen is rarely convinced to engage in any adventurous chemical bonding. With a triple bond connecting the two nitrogen atoms, it is quite happy as it is.
Yet nitrogen compounds are fundamental to all life on Earth. They include nucleic acids and proteins, and the energy-carrying molecule adenosine triphosphate, or ATP. Almost all nitrogen fixation – converting N2 to some kind of compound, usually ammonia – is done by bacteria that contain nitrogenase.

Nitrogenases are mysterious enzymes. They are extremely oxygen-sensitive, so the bacteria that harbour them take great care not to expose them to the gas. Most nitrogen-fixing bacteria live in soil, some in symbiosis with plants. Pea plants, for example, have special root structures where bacteria are housed in a low-oxygen environment in exchange for life-giving nitrogen compounds .
Chemists have been fascinated with nitrogenase’s inner workings for a long time. And still, after almost 100 years of research, they are not quite sure how the enzyme does its nitrogen-fixing magic.
Here’s chemist Ross Milton, from Stanford University, talking about what scientists have already found out:
Ross Milton
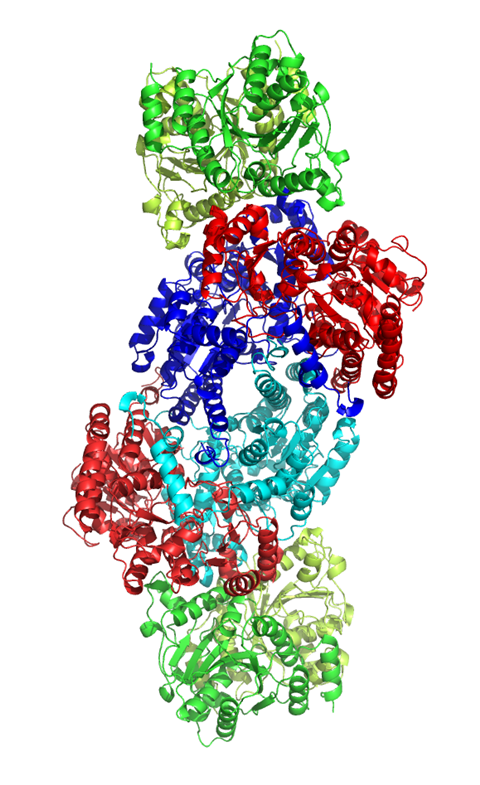
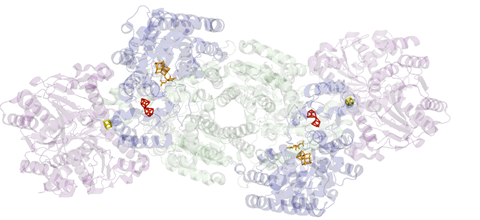
Nitrogenase is actually a di-protein, or two-enzyme, assembly. It has a central catalytic protein, which is where N2 is reduced to ammonia, and it’s all satellited by a reductase. This protein delivers electrons to the catalytic protein. When these two transiently associate, an electron is transferred, one at a time, from this reducing protein to the catalytic protein. With that, we know that ATP has to be hydrolysed to enable that transfer but we don’t exactly understand the role of ATP hydrolysis and how this facilitates activity.
Katrina Krämer
But that’s only one electron transfer. Overall, eight of them must happen to accumulate enough electrons to fix N2 to ammonia. For each ammonia molecule produced, the enzyme chomps up a whopping 16 of ATP, a high price in the biological energy currency. The fact that fixing nitrogen requires so much energy yet bacteria still do it shows just how important the process is.
The actual nitrogen fixation happens within nitrogenase’s metal heart. The enzyme houses a cluster of iron and molybdenum atoms called FeMoco (for iron–molybdenum cofactor).
Ross Milton
How it’s reduced is still under debate. The field agrees that there’s a critical state called the E4 state, which is a four-electron reduced FeMoco – in the molybdenum case – at which N2 has to then interact with the co-factor before being reduced to ammonia. But how that proceeds, we don’t actually know.
Katrina Krämer
The point of contention is the metal itself. Many eminent chemist have debated whether it’s the iron or the molybdenum that does the catalysis. But it is a debate worth having. If scientists could find a catalyst that gets even close to unpicking the nitrogen triple bond in the same way nitrogenase does, it could rival the world’s most energy-consuming reaction: the Haber–Bosch process.

Ross Milton
In total, we produce about 500 million tons of ammonia per year and the majority of this comes from the Haber–Bosch process. In order for the process to be efficient – which it is – it requires high temperatures, about 400°C, and high pressures, 200 atmospheres – about a hundred times greater pressure than in a typical car tyre. As a result, the entire process requires about 1% of all of the energy produced on Earth and it’s thought to be responsible for about 3% of CO2 emissions.
Katrina Krämer
Although the Haber–Bosch process eats up enormous amounts of energy, it is humanity’s essential source of fertilisers. Crops grown with synthetic fertilisers derived from ammonia feed half of the people on the planet. And it is still the best process we have. No other process is better at prying apart nitrogen than this 100-year-old chemical sledgehammer.

Ross Milton
The Haber–Bosch process does require a lot of energy, but it is great at what it does. Each pass of N2 fixation to ammonia in the Haber–Bosch process is only about 15% efficient, but overall, by recycling those gases through the system, it reaches over 95% efficiency. It’s very good at what it does. But I think the future is decentralising the Haber–Bosch process so that we don’t have to have these massive pressure reactors and these ammonia production plants located at select sites across the globe. If you can make that ammonia without high-pressure equipment on your farm, then we could improve environmental sustainability.

Katrina Krämer
For Milton and a team around Shelley Minteer from the University of Utah, US, this was incentive enough. But instead of creating fancy new metal catalysts, they harnessed nature’s ammonia factory.
The team extracted nitrogenase from bacteria and stuck it to an electrode. Together with a hydrogen-oxidising enzyme stuck to another electrode, the setup converts a mix of hydrogen and air into ammonia – at room temperature and ambient pressures. And not only that, the reaction also produces a little bit of electricity in the process.
Ross Milton
It is only a prototype system at the moment, it is very low scale. It compares in that it can do N2 fixation at ambient pressure and temperature, and demonstrates that it’s possible to recover a small amount of electrochemical energy from the same process. It does require ATP hydrolysis, which is still chemically quite expensive. So one way forward will be to engineer nitrogenase or the system such that it doesn’t require the hydrolysis of ATP.
Katrina Krämer
Whether this biological fuel cell is the way forward for ammonia synthesis remains to be seen. Although nitrogenase is still the master of fixing lazy nitrogen into life-saving fertiliser, for now, the Haber–Bosch process is here to stay.
Ben Valsler
That was Katrina Krämer speaking with Ross Milton. Thanks again to Simon Bayly for the suggestion! Next time, Mike Freemantle poses a chemistry question:
Mike Freemantle
Here’s a quiz question for you. What links the Italian Renaissance artist Leonardo da Vinci, 18th century German composer Johann Sebastian Bach, oak trees and ferrous sulfate?
Ben Valsler
Let us know what you think and join Mike next week to find out. Until then, get in touch in the usual ways – email chemistryworld@rsc.org or tweet @chemistryworld. Thanks for listening, I’m Ben Valsler.












No comments yet