Lignin, a component of plant cell walls, gives plants the strength to grow tall but this strength is a barrier to turning plants into chemicals. So researchers in the UK have devised an efficient way to make complex model compounds of lignin to help them figure out the best way to break lignin down.
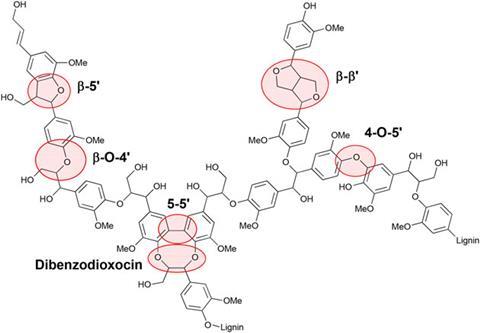
Unlike cellulose, a plant cell wall component with a repeating polymer structure, lignin is a complex and random polymer. The chemical units are linked by different connectivities, so one single process cannot attack all of these bonds. Previously, monomers and dimers were used to model chemical linkages of lignin, but were too simple for the study of lignin itself. More complex trimers, tetramers and hexamers have been synthesised, but with inefficient, low-yielding methods.
Work undertaken in Gary Sheldrake’s group at Queen’s University Belfast looks set to significantly advance the study of lignin with the development of a new scalable synthetic route for complex model lignin oligomers that produces several grams of product, and can easily be performed in a standard lab. ‘The ultimate aim was to get a synthesis that worked, but we tried as far as possible to avoid ungreen solvents and harsh conditions,’ notes Sheldrake.
Biomass to biofuel conversion expert, Bert Weckhuysen, from Utrecht University in the Netherlands, believes this achievement will allow investigation into how the oligomer structure influences the ease with which a catalytic scissor can cut the different linkers within lignin.
Gill Stephens, who studies enzymatic lignin depolymerisation at the University of Nottingham in the UK admits to previously struggling due to the lack of good model compounds. ‘This is really good as you have all of the lignin bonds sitting there nicely, which offers a fantastic opportunity to begin to understand and control the depolymerisation process and to find out how to stop enzymes polymerising lignin instead.’
Sheldrake says they are ‘beginning to determine what is important in terms of designing chemical methods for breaking lignin down into useful chemicals,’ and hopes that other researchers will be able to build on this work to produce larger model compounds that resemble lignin even more closely.






No comments yet