Carbon dioxide produced by human activity is acidifying the ocean at an unprecedented and alarming rate. Nina Notman investigates

The home to 80% of the Earth’s organisms is currently under attack from global warming’s ‘evil twin’: ocean acidification.The gases in the atmosphere and dissolved in the ocean are in equilibrium, meaning that as the carbon dioxide concentrations in the atmosphere increase, those in the ocean do too. Approximately 27% of the carbon dioxide released from burning fossil fuels and deforestation since the industrial revolution has been taken up by seawater.
Initially this was thought to be a good thing, as removing some of the anthropogenic carbon dioxide from the atmosphere limits its ability to warm up the planet. But about 15 years ago scientists realised that this excess carbon dioxode is changing the carbonate chemistry of the ocean. ‘When you add carbon dioxide to water it forms carbonic acid,’ explains Carol Turley, a senior scientist at Plymouth Marine Laboratory, UK, who coordinates the UK Ocean Acidification Research Programme. ‘Then it goes through a series of other chemical reactions which result in a release of hydrogen ions making the pH decrease,’ she adds.
‘The pH has decreased by 0.1 of a unit [from 8.2 to 8.1] since the industrial revolution,’ says Turley. ‘It’s a logarithmic scale, meaning an approximately 30% increase in acidity.’
The term ocean acidification is also used to cover the other impacts that increasing carbon dioxide concentrations are having on the ocean and marine organisms. This includes an increase in bicarbonate ion (HCO3–) concentrations and a decrease in that of carbonate ions (CO32–) that happen in seawater as a result of the cascade of reactions that occur when excess carbon dioxide dissolves in it. The reduction of carbonate ions, required to build the calcite and aragonite polymorphs of calcium carbonate, is of particular concern as many marine organisms require them to keep shells and skeletons intact. Damage to some calcium carbonate based marine organisms, such as coral reefs, oyster larvae and sea snails, in carbon dioxide enriched water is already being seen in some parts of the world.
Learning from the past
In the history of the Earth, atmospheric and therefore oceanic carbon dioxide levels have previously been very high. Therefore the pH of the ocean has previously been very low and the carbonate chemistry balance different to today. ‘What is unprecedented – as far as we know – in the past 65 million years is the rate of change,’ explains organic geochemist Daniela Schmidt from the University of Bristol, UK. It has been estimated that ocean acidification is currently occurring nearly 10 times faster than at any time in the past 65 million years.
Ocean chemistry is naturally buffered by mechanisms such as the dissolution of carbonates deposited on the sea floor. But these mechanisms can’t keep up with the current rate of change. ‘If it was happening over more than 10,000 years, the Earth’s systems would buffer it up,’ says Turley. ‘What is happening now won’t be buffered out quickly; it is going to be around for tens of thousands of years.’
The rate of change is also of great concern for the future of certain marine organisms, as it needs to be slow enough for them to evolve and thrive under the new conditions. ‘For an ecosystem and its adaptation ability, the rate of change is fundamentally important,’ says Schmidt.
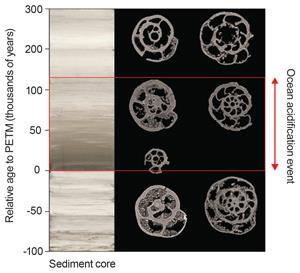
A number of scientists, including Schmidt, are looking at fossils of organisms that were alive during past ocean acidification events to help predict how the ecosystem will react this time. ‘We look at what the ecosystem reaction was [to previous acidification events] and see what we can learn from that,’ she says. The rate of pH change can be determined by analysing the ratio of boron isotopes in fossils of different ages. Changes in calcification that occurred during these events can also be assessed and whether certain species went extinct can be determined. A period of time about 56 million years ago, known as the Paleocene–Eocene Thermal Maximum, is of particular interest as it was the last time the Earth’s carbon dioxide levels rose sharply.
‘The most exciting thing we have done is taken fossils to the synchrotron in Switzerland,’ says Schmidt. These fossils of foraminifera alive during the Paleocene–Eocene Thermal Maximum, and another slightly less dramatic global warming event 53 million years ago, were drilled out of a seabed off Namibia. This species of single-celled organisms still exists in the ocean today. Using synchrotron tomography, Schmidt’s team reconstructed the shell thickness of the fossilised organisms. They then obtained pH values for the same specimens. ‘We took one fossil to tell us the whole story,’ she says.
The results of this study were counter-intuitive: thicker walls were observed during times with the lowest pH. ‘This suggests that some species have coping mechanisms by increasing calcification with cost for other physiological parameters,’ she says. ‘The main message that came out of this work is that if you [acidify the ocean] comparatively slowly, the ecosystem just adapts to it. If you do it very, very rapidly we can see extinction.’
Long-term time series

Since 1988, direct measurements of seawater pH, dissolved inorganic carbon concentrations and total alkalinity have been taken on a near-monthly basis at the same location in the Atlantic Ocean and another in the Pacific Ocean. These values are then used to calculate the partial pressure of carbon dioxide (pCO2), and the bicarbonate and carbonate ions concentrations.
The Bermuda Atlantic Time Series Study (BATS) and the Hawaii Ocean Time Series (HOT) have both recorded a significant increase in acidity and pCO2 of the surface seawater in their sampling area since measurements began.
The sampling location for the HOT programme is Station Aloha (the conveniently named long-term oligotrophic habitat assessment), 115km north of Oahu island in the US state of Hawaii, explains team member Roger Lukas. He has worked on this time series since its initiation. ‘We chose that site to be representative of the vast deep ocean area around Hawaii,’ he says.
Approximately once a month, a research ship spends four days collecting samples and data at this site. The team are measuring around 25 core parameters each trip for a variety of different oceanic research purposes. The water samples needed for the ocean acidification research are collected using a rosette sampler that holds 24 bottles, each holding 12 litres, explains Lukas. The sampler is lowered on a cable and the bottles can be opened and closed remotely from the lab onboard the ship. ‘Approximately 16 casts are made at Station Aloha on each cruise,’ he says.
‘We collect a full vertical profile from the surface all the way down to near the bottom of the ocean, which is about 4850m at Station Aloha,’ explains Matthew Church, the HOT programme’s principal investigator. ‘We collect samples at 25 to 30 discrete depths and measure seawater pH, dissolved inorganic carbon concentrations and total alkalinity at all of those different depths.’
The pH is measured onboard the ship using a pH-sensitive dye and a spectrophotometer. The samples for dissolved inorganic carbon and total alkalinity are preserved at sea to halt biological activity, and then analysed in a laboratory on dry land.
To obtain a broader view of how the oceans’ pH and carbonate chemistry are changing over time, the US National Oceanic and Atmospheric Administration (NOAA) is running a large-scale ocean acidification observation programme. ‘We have 38 mooring stations throughout the world,’ explains Richard Feely, a marine chemist at NOAA’s Pacific Marine Environmental Laboratory in Seattle. The sensors on these autonomous moorings take continuous measurements of chemical and physical parameters. ‘The mooring data gives us high resolution time series at fixed locations,’ he says. These moorings are located along the US coasts and in the open ocean.
NOAA also runs regional research cruises taking measurements of the same parameters along the US’s east, west, gulf and Alaskan coasts. These cruises are repeated every four years. ‘We go to approximately 100 stations in each of these regional cruises,’ says Feely. ‘So we get very detailed information on how the conditions are changing.’
Feely is also co-chair of the US contribution to the global GO-SHIP programme, which coordinates a series of global research cruises. Each of these cruises travels across long sections of the global ocean and are repeated approximately every 10 years, he explains. Each cruise collects samples at around 200 locations, approximately 35 miles apart. ‘We have been doing the repeat hydrography cruises since the late 1980s, and so we have been able to see changes to the chemical composition of the ocean on a decadal timescale,’ says Feely.
Impact on marine life

Marine organisms in the US west coast, African west coast and the west coast of Peru are already showing signs of struggling with the rise in water acidity in these regions. In these locations wind-driven ocean currents cause deeper water layers – that are naturally higher in carbon dioxide than surface water layers – to come up to the surface. The combination of this natural upwelling process and anthropogenic ocean acidification in these regions is pushing the pH of the water down low enough that negative effects on organisms are starting to be seen. This allows scientists a glimpse into the future of how ecosystems worldwide may behave if global oceanic carbon dioxide levels continue to rise.
And it isn’t good news. In 2007, larvae at oyster hatcheries on the US west coast began dying at an unprecedented rate, pushing a multimillion pound industry to the brink of collapse. This problem was traced back to local changes in ocean acidity. Efforts to monitor seawater pH, and to close the flow of water into oyster hatcheries when the pH starts dropping, are now alleviating the issue.
Meanwhile, Feely and his colleagues collected evidence of dissolution of calcium carbonate shells of marine snails known as pteropods during a cruise of the US west coast in 2011. Marine snails were collected by net, and the pH and other relevant parameters measured, at various locations in this region. At sampling sites with acidified waters, severe dissolution was observed. ‘We are seeing acidification effects in the ocean right now in marine pteropods,’ says Feely. ‘If shells [of living marine organisms] begin to dissolve, it is a threat to the survival of those organisms and therefore a threat to the ecosystem because they are an important food source for higher-level organisms.’
However, it is not simply the decreased pH of the seawater that is causing these snail shells to dissolve, nor the death of the oyster larvae, nor any other negative effects of ocean acidification on marine organisms that have been reported. ‘We cannot say that all ocean acidification effects in organisms are caused by water pH changes – that is too much of a simplification,’ says marine biologist Hans-Otto Pörtner at the Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research in Bremerhaven, Germany. When carbon dioxide diffuses from the ocean into marine organisms it causes similar chemical changes to those occurring in the seawater itself. Its concentration in the blood increases, blood pH and carbonate concentration decreases, says Pörtner. ‘It’s a complex picture that results.’ The reduction in carbonate ion availability in body fluids hinders the ability of snails to rebuild their shells for example, and the extra carbon dioxide impacts on various different internal systems within the organisms. Other factors related to global warming, such as rising water temperatures, also add to the complication.

In 2011, Pörtner and his colleague conducted a meta-analysis of 167 studies into different species’ sensitivity to a range of carbon dioxide concentrations in seawater. They looked at five animal groups – corals, crustaceans, molluscs, fish and echinoderms such as starfish and sea urchins – and found that they all suffer at high carbon dioxide levels, but by varying amounts. Different species have different capacities to defend against, and compensate for, ocean acidification, explains Pörtner. And it is this adaptation capacity that will ultimately determine which species survive the current rate of pH change. Pörtner’s meta-analysis showed that warm water corals, molluscs and echinoderms are more sensitive to high carbon dioxide concentrations than fish and crustaceans.
But for organisms that depend exclusively on bicarbonate ions, and don’t need carbonate ions, many may thrive in more acidic waters. ‘The bicarbonate might make it easier for some phytoplankton or macroalgae to photosynthesise,’ explains Turley. ‘However, some species doing well isn’t always good for an ecosystem.’
Looking to the future
It is thought that 24 million tonnes of carbon dioxide is absorbed by the ocean each day. And it has been predicted that, if fossil fuels continue to be burnt and forests cut down at the current rate, the ocean’s pH will decrease approximately 0.3 units further by the end of the century. ‘If we keep on business as usual, ocean acidity will increase by 100–150% by 2100,’ says Turley.
All the evidence suggests that this will result in marine ecosystems becoming less diverse, she says. ‘One billion people on the planet depend on marine protein as their sole protein source and we need to start questioning whether the ocean can continue to provide the same food quality.’
Reducing the amount of carbon dioxide in the atmosphere, and therefore the ocean, is the only way to halt this change in ocean acidity. ‘If we want to stop acidification, or slow it down, we have to reduce carbon dioxide emissions,’ says Turley. Geoengineering approaches that remove carbon dioxide from the atmosphere may also be able to offer a helping hand.
The most promising of these is carbon capture and storage, she says. This would involve capturing the carbon dioxide emitted by oil refineries and fossil-fuel-fired power stations and injecting it back into oil and gas wells. Cost concerns, political wranglings and concerns about future leakages from the storage sites are hampering development of this technology at present. ‘There are also questions about how much capacity we really do have and what is technically possible, but that’s an exciting engineering challenge,’ says Schmidt, who is also hopeful for the future of other geoengineering approaches.
‘If you think about all the engineering changes that have taken place in the world over the past 100 years, if we were to invest this energy capacity to combat climate change and ocean acidification, I’m pretty sure we can.’
Nina Notman is a science writer based in Salisbury, UK











No comments yet