Our gut bacteria are carrying out chemistry on our behalf, but without us knowing much about it. Scientists are starting to examine their enzymes, find out what they’re up to, and see if they can be harnessed to help us
It’s well known that the vast majority of drugs – up to 80% – are metabolised by just one enzyme family in the liver, cytochrome P450. But what about enzymes belonging to the other organisms in our body – the billions and billions of bacteria in our digestive system’s ‘microbiome’ – how do they affect the drugs we take? And what impact do they have on our diets? Chemists and biologists are beginning to home in on gut bacterial DNA, linking the genes to important enzymes, metabolites and health effects.
It may soon be possible to screen an individual’s microbiome for particular bacterial genes or metabolites to estimate how well they will respond to a drug before deciding on a dose. Drug outcomes could also be improved by giving targeted bacterial supplements, and mechanistic insights could even help to develop drugs that bacteria are unable to inactivate.
In the US, the Environmental Protection Agency and the National Institute of Environmental Health Sciences are concerned about the human microbiome possibly altering the way our bodies respond to environmental chemicals. They recently asked the National Academy of Sciences to put together an expert committee for a research strategy to improve understanding of how environmental chemicals can interact with the human microbiome. The group warned that ignoring chemical–microbiome interactions could lead risk assessors to over- or under-estimate the possible human health effects resulting from chemical exposure.
Culture club
Much of the science surrounding gut microbial metabolism is decades old yet was published at a time when analytical techniques were much less sensitive, and many genetic techniques did not exist. So in many cases it is known that a drug or nutritional compound is broken down in the gut, but scientists have yet to pin down the bacteria responsible, the enzymes involved or the metabolites generated. Advances in DNA sequencing technology, together with sensitive mass spectrometry and NMR techniques, are now allowing researchers to revisit old studies and tease out the mechanistic details.
There is so much diversity and redundancy in what bacteria do
It is critical to connect the vast number of uncharacterised genes in the human gut microbiome to biochemical functions, says Emily Balskus from Harvard University in the US. The chemistry is ‘very important – potentially more important than the bacterial species identity’, she adds. ‘Knowing what a microbe is doesn’t necessarily tell you much about what it can do, because the genomes of a given species will be so diverse. The functional capabilities are what we may need to really focus on when we think about trying to elucidate how bacteria are interacting with the host.’
In addition, screening for metabolites can help to reveal which microbially derived molecules might be linked to which disease. ‘It doesn’t confirm that they have a causative role in disease but it can be a way to prioritise metabolic activities for further studies,’ she says.
To complicate matters, there is a fair amount of genetic ‘redundancy’ in the microbiome, with different bacteria having the same function, says Karen Scott from the University of Aberdeen in Scotland. A change in the composition of gut bacteria doesn’t necessarily indicate functional changes. ‘There is so much diversity and redundancy in what bacteria do. It’s not ever going to be simple with such a complex ecosystem,’ she says. Not to mention the fact that everyone has a different microbiome.
In most cases, researchers can grow bacteria in a lab, under anaerobic conditions. It is also possible to study the systems using germ-free mice, which are animals devoid of microorganisms that can be colonised with a particular bacterium to see if a suspected metabolic activity really occurs. Meanwhile, human faecal samples can be incubated with dietary compounds or drugs before studying the metabolic landscape.
The drugs don’t work
Human gut microbes are known to transform over 50 pharmaceuticals into metabolites with different pharmacological properties. Such transformations can activate or inactivate a drug or even cause toxicity, but in most cases the specific bacteria and enzymes involved are unknown.
‘One of the humbling things that we have learned is that the microbiome is potentially relevant to all of the major organs in the body,’ says Peter Turnbaugh from the University of California, San Francisco in the US. ‘We have a long way to go before we understand all of the various ways in which the microbiome can affect our response to drugs,’ he adds.
Turnbaugh is particularly interested in whether the microbiome is ‘specialised or generalist’. Human enzymes, such as cytochrome P450s, which metabolise thousands of chemicals, have evolved to be able to accept many different substrates. ‘And so the question for the microbiome is whether microbes can do the same thing or whether they have separate enzymes for every type of drug they could metabolise,’ he says.

In recent years, Turnbaugh has focused much of his attention to exactly how gut microbes break down digoxin, which he says is a textbook example of a drug that is affected by the microbiome. A cardiac drug, digoxin was originally derived from foxglove extracts and used for centuries to treat congestive heart failure, otherwise known as dropsy. The first scientific papers on the effectiveness of foxglove extracts were published by two competing medics in 1785. The active constituent is a glycoside, which works by inhibiting enzymes that rely on sodium and potassium ions.
These days, digoxin is used to treat certain types of irregular heartbeat but the drug has a very narrow therapeutic window. This can be explained by the fact that about 10% of humans have gut bacteria that inactivate digoxin by reducing it to the inactive dihydrodigoxin. In some cases, bacteria can inactivate over half of the administered drug.
In the 1980s, responsibility was pinned to a single bacterial species called Eggerthella lenta. That was as far as the knowledge went until decades later, when Turnbaugh’s team used RNA sequencing technology to identify a gene cluster only present in a digoxin-metabolising E. lenta strain. Assays showed that a dietary amino acid called arginine could halt digoxin’s inactivation. Indeed, the researchers have suppressed inactivation by feeding an arginine-rich diet to mice with a digoxin-reducing strain of E. lenta.1
Turnbaugh hopes to be able to identify general drug metabolism trends in the human population. ‘For example, with digoxin we find that you can sort of group people based on the abundance of the glycoside enzyme,’ he says. ‘It is not completely binary but it is possible to stratify microbial communities from samples based on people with high and low levels of the gene,’ he says. ‘Potentially you could use the information as a better way to optimise drugs. What we really want to move towards is being able to profile the patient before they start taking a drug, and then predict what the best dose may be for that patient or a group of patients with a similar microbiome.’
Together with US informatics experts including Russ Altman from the Food and Drug Administration and Emily Mallory from Stanford University, Turnbaugh is also working on a computational way to predict drug metabolism by the human gut microbiome.2 Understanding the ‘complete space of drugs’ that are metabolised by the human gut microbiome is critical for predicting bacteria–drug relationships and their effects on a patient’s response, they suggest.
They begin with a known drug metabolism such as digoxin’s. Rather than look for other chemicals that are structurally similar, the program hunts out related reactions. With more data, the team hope their technique could allow researchers to screen drugs and their metabolites against chemical reactions common to gut bacteria.
Harvard’s Balskus would like to see pharmaceutical companies routinely screen gut microbial samples or individual microbial strains of enzymes for potential drug metabolism, in the same way that they screen drugs against a panel of human liver enzymes. She is keen to see more chemists and chemical biologists get involved in the field to develop chemical tools, such as probes that could selectively interact with individual microbial metabolic activities.
Food for thought
Balskus’s lab also investigates into how microbes interact with dietary nutrients. ‘The microbiome really influences the nutritional content of our food in ways that are not understood,’ she says.
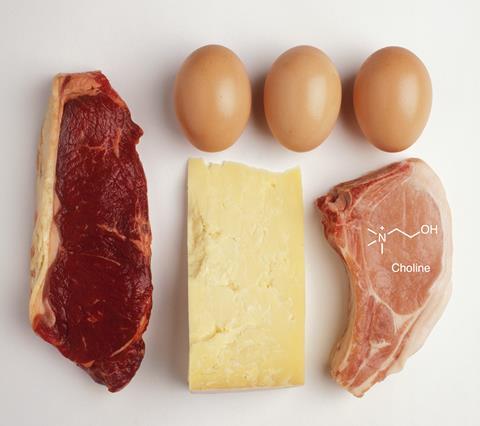
In general, gut bacteria mainly break down proteins and carbohydrates from food. For example, they ferment indigestible dietary polysaccharides to produce short chain fatty acids (SCFAs), including acetate, propionate and butyrate. Butyrate is thought to be important for protecting against colon cancer and keeping the gut epithelial layer growing and turning over, says Scott. The metabolites are also known to be energy sources for cells in the body, as well as being precursors and regulators of molecules such as cholesterol, fatty acids and glucose.
But choline, a nutrient found in meat, eggs and milk, can have less desirable metabolites. The ammonium salt is a precursor molecule for the neurotransmitter acetylcholine, which is involved in memory and muscle control. However, gut microbes can break the nutrient down into trimethylamine (TMA), which is then converted to trimethylamine N-oxide (TMAO) in the liver. Studies have found a strong correlation between increased blood levels of TMAO and the risk of developing heart disease and non-alcoholic fatty liver disease.
In 2012, Balskus and fellow Harvard researcher Smaranda Craciun identified the gene cluster responsible for degrading choline in the genome of a sulfate-reducing bacterium.3 The choline utilisation (Cut) cluster led researchers to a key TMA-forming enzyme called choline TMA-lyase (CutC). Subsequent searches for Cut genes revealed that the function is widely distributed in human gut bacteria.4
Other groups have since developed gut communities with decreased TMA production and small molecule inhibitors, which could lead to potential therapeutics for treating associated adverse health effects.
Pass the coffee
‘Better understanding of microbe–dietary interactions could help to explain the health benefits associated with certain foods,’ suggests Balskus. For example, dietary polyphenols such as anthocyanins have long been associated with health benefits. ‘It turns out that they are not actually readily absorbed in the gut. They stay in the gastrointestinal tract and are transformed by gut microbes,’ she says.
At Pennsylvania State University in the US, Jairam Vanamala is using a metabolomic approach to study how polyphenolic compounds such as anthocyanins can reduce inflammation in the gut, possibly lowering the risk of developing inflammation-promoted chronic diseases such as colon cancer and type two diabetes.

The researchers fed pigs a high-calorie diet supplemented with raw, baked or fried purple potatoes, which are rich in anthocyanins. ‘We fed the pigs a high-calorie diet and saw that it can promote colonic inflammation,’ says Vanamala. ‘This is important for two reasons: it drives carcinogenesis and may be responsible for a leaky gut.’
Using liquid chromatography–MS/MS-based proteomics, the team confirmed that pigs fed a high-calorie diet supplemented with baked or raw purple potatoes had lower levels of a protein linked to inflammation called interleukin-6 (IL-6).5 Vanamala’s team is now looking at which gut bacteria are elevated or suppressed by the high-calorie diet at the point when inflammation sets in, as well as tracking up to 5000 metabolites.
Although the results have yet to be published, they suggest that levels of a particular bifidobacterium go down with a high-calorie diet. This is interesting because some bifidobacteria release esterase enzymes that are important for hydrolysing phenolic acids called chlorogenic acids into smaller molecules (quinic acid and caffeic acid) with potent anti-inflammatory activity, explains Vanamala.
He has a theory that certain people may lack specific bacteria that are able to cleave the polyphenols into smaller compounds, giving them less ammunition to fight inflammation. ‘If we supplement their diets with bacteria then we may see levels of small, more potent gut-bacteria derived polyphenol metabolites go up,’ he suggests.

Next, Vanamala would like to study whether drinking coffee, which is naturally rich in polyphenols, including chlorogenic acids, can protect against the negative effects of a high-calorie diet. Vanamala and Penn State colleague Lavanya Reddivari are also studying well-known food emulsifiers, after US research showed that carboxymethylcellulose and polysorbate-80 may promote colonic inflammation in mice. The emulsifiers are commonly added to foods such as ice cream to enhance texture and extend shelf-life. In mice, the emulsifiers can reduce the thickness of intestinal mucus, according to research published in 2017 by a team led by Benoit Chassaing from Georgia State University.6
The Georgia researchers used a mucosal simulator of the human intestinal microbial ecosystem (M-SHIME) to show that the emulsifiers directly alter gut microbiota to increase the potential to cause inflammation. They plan to carry out human clinical trials on dietary emulsifiers and suggest that models like M-SHIME may be a useful way to develop food additives that don’t have a negative effect on the microbiome.
Vanamala and Reddivari have also fed the emulsifiers to mice. ‘We saw similar inflammation but also used polyphenolic compounds to see whether we can counter emulsifier-induced colonic inflammation,’ he says. Some foods that are rich in polyphenols seem to suppress the inflammation, he says. So one tasty solution to possible emulsifier-induced inflammation in humans could be to add polyphenols. Berries and ice-cream, anyone?
Food on the brain
One of the ‘hottest areas in microbiome research’ involves the so-called gut–brain axis, says Jonathan Swann from Imperial College London in the UK. Some small molecules in the gut travel across the blood–brain barrier to affect appetite or mental state. For example, acetate produced by gut bacteria can travel to the brain in mice, where it can modulate appetite, according to a 2014 study led by Gary Frost, also at Imperial.7 The team has also shown that SCFAs can act in the gut to release hormones that affect hunger.
The microbiome is a fun place to be right now and there are a lot of opportunities for chemists
Swann is part of a team working on how the microbiome might affect depression. The researchers have shown that delaying weaning or prolonging exposure to breast milk in rats can result in a depressive-like phenotype.8 They found that rats weaned at 25 days of life had significant differences in metabolic profiles compared with those weaned at 21 days. Such changes were consistent with the metabolic signatures seen in depressed humans. The researchers suspect that the answer lies in a milk protein called casein, which is broken down by host enzymes and bacteria in the gut to liberate compounds called beta-casomorphins, which have opioid-like properties.
The team has since repeated the experiments, but with added antibiotics, and is currently exploring how the findings may translate to humans. ‘Suppressing the bacteria with antibiotics prevents the liberation of beta-casomorphins and we lose the depressive effects, providing evidence that the bacteria are involved,’ says Swann.
Studying the zoo of microbes, enzymes and metabolites within our digestive system is clearly an important and expanding area of research. ‘It’s a fun place to be right now and there are a lot of opportunities for chemists and chemical biologists. Microbes are interacting with small molecules and they are making small molecules,’ says Balskus. ‘Figuring out how this varies between people and what the biological consequences of gut microbial chemistry are will be really important and essential to transform our views of what it is to be human.’
Emma Davies is a science writer based in Bishop’s Stortford, UK
References
1 H J Haiser, Science, 2013, 341, 295 (DOI: 10.1126/science.1235872)
2 E K Mallory et al, Pac. Symp. Biocomput., 2018, 23, 56 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5771676/
3 S Craciun & E P Balskus, Proc. Natl Acad. Sci USA, 2012, 109, 21307 (DOI: 10.1073/pnas.1215689109)
4 S Bodea et al, Cell Chem. Biol., 2016, 23, 1206 (DOI: 10.1016/j.chembiol.2016.07.020)
5 A Sido et al, J. Nutrit. Biochem., 2017, 43, 11 (DOI: 10.1016/j.jnutbio.2017.01.012)
6 B Chassaing et al, Gut, 2017, 66, 1414 (DOI: 10.1136/gutjnl-2016-313099)
7 G Frost et al, Nat. Commun., 2014, 5, 3611 (DOI: 10.1038/ncomms4611)
8 P Farshim et al, Sci. Rep., 2016, 6, 21958 (DOI: 10.1038/srep21958)












No comments yet