Wild fires adversely affect air quality nearby and far beyond. Nina Notman investigates this escalating problem
Millions of tonnes of toxic gases and smoke particles are spewed into the atmosphere by forest fires and other wildfires every year, making them a major source of global air pollution.
In October 2017, for example, more than 10,000 tonnes of fine particles were reportedly emitted during an extreme week of wildfires near the San Francisco bay area in the US state of California – more than vehicles in the state would normally emit in a year. And across the US, burning vegetation causes a fifth of the occurrences each year where fine particle concentrations exceed the safe levels set by the Environmental Protection Agency.

These emissions have a major impact on global human health. The 2015 haze across Asia, caused by an anomalous year of fires in Indonesia, was estimated to cause over 100,000 premature deaths in the region.
The increasing occurrence and size of wildfires means that air pollution from this source is on the rise. Particulate matter air quality has improved everywhere in the US in recent decades due to reductions in car and power plant emissions – except for wildfire-prone areas. Similar patterns have been reported in Australia.
Smoke impact is inevitably largest near the fires, but some of the pollutants travel in the atmosphere for thousands of miles. In November 2018, a model showed that Californian wildfire smoke was contributing to a New York City haze. And the UK is affected by pollution from Canadian wildfires drifting across the Atlantic.
A complex puzzle

In the summer of 1978, Dutch chemist Paul Crutzen was flying around northeastern Colorado collecting atmospheric samples from livestock farms, when a wildfire caught his eye. ‘We saw a big forest fire high up in the Rocky Mountain National Forest, which provided us with the opportunity to collect air samples from a major forest fire plume,’ Crutzen explained during his lecture after receiving the 1995 Nobel prize for chemistry. Chemical analyses back on the ground determined carbon monoxide, nitrogen oxides and a handful of other trace atmospheric gases emissions from that fire. This data was then used to estimate global wildfire emissions. ‘Biomass burning has previously been considered unimportant as a global source of atmospheric trace gases – our analysis shows that this is not the case,’ his 1979 Nature article on the work concluded.
These first impromptu measurements stimulated considerable interest in the chemical composition of wildfire smoke. Over 40 years later, it’s now known that smoke contains several thousand different compounds. ‘When you burn the cellulose and lignin – the complex polymers that make up plants – and the other compounds that are in vegetation, it produces a myriad of chemical species that go into the atmosphere,’ explains Jose Jimenez, an atmospheric chemist at the University of Colorado Boulder in the US. These vary considerably between fires, with the chemical makeup of the fuel being a key factor.
The moisture content of the vegetation is also important. ‘As the environment dries out during the dry season, more parts of the ecosystem tend to burn,’ says Bob Yokelson, an atmospheric chemist at the University of Montana in the US. ‘This creates a more complex, more polluting mixture than if the fire had occurred when the ecosystem was moister.’
Likewise, smoke composition varies during the different stages of a fire. At the start, before the fuel reaches its flashpoint, organic gases evaporate off and white-coloured organic particles form. Then, once alight, ‘provided there is sufficient oxygen, the fuel burns quite efficiently, producing tiny black carbon [soot] particles and the organic emissions drop quite substantially’, explains Hugh Coe, an atmospheric scientist at the University of Manchester, UK. The final – longest – stage is smouldering, a very inefficient and highly polluting form of combustion with less soot and more organic matter released. ‘The organic matter in the smouldering phase is not the same composition as the organic matter in the initial pyrolysis phase,’ Coe adds. An added complication is that all three stages take place side by side in a wildfire.
In addition, many released compounds react with each other, and other things in the atmosphere, while travelling downwind. ‘The species are not inert,’ Jimenez says. ‘They’re going to change when they’re in the atmosphere; they’re going to be oxidised, some of them are going to go from a gas into a particle form, some of them turn from particles to gases.’ Yokelson adds: ‘Smoke analysis is about as challenging a mixture analysis problem as you can imagine.’
Modelling the future
Better protection of human health is a primary motivation for studying the chemistry of smoke. Particles (known as particulate matter, PM) are the largest concern. Many epidemiological studies have demonstrated that a higher concentration of these in the atmosphere correlates with higher levels of premature mortality. Particles smaller than 2.5µm, PM2.5, are a particular concern as they can travel further into the respiratory system.
There is also evidence that smoke gases react with other air pollutants to produce another major air pollutant and respiratory system irritant – ozone. Other components of smoke, such as polycyclic aromatic hydrocarbons (PAHs), are suspected to be less of a concern. ‘Not even the fire fighters are generally exposed to toxic gases at levels that are above the legal thresholds for what would be allowed in an industrial setting,’ explains Yokelson. But these legal limits only consider chemicals in isolation. The potential impact of combinations of toxic gases on the body remains poorly understood. ‘By studying the chemistry, we can get a better idea of what people are being exposed to and how it compares to other exposures that they might get [via other means],’ he adds.
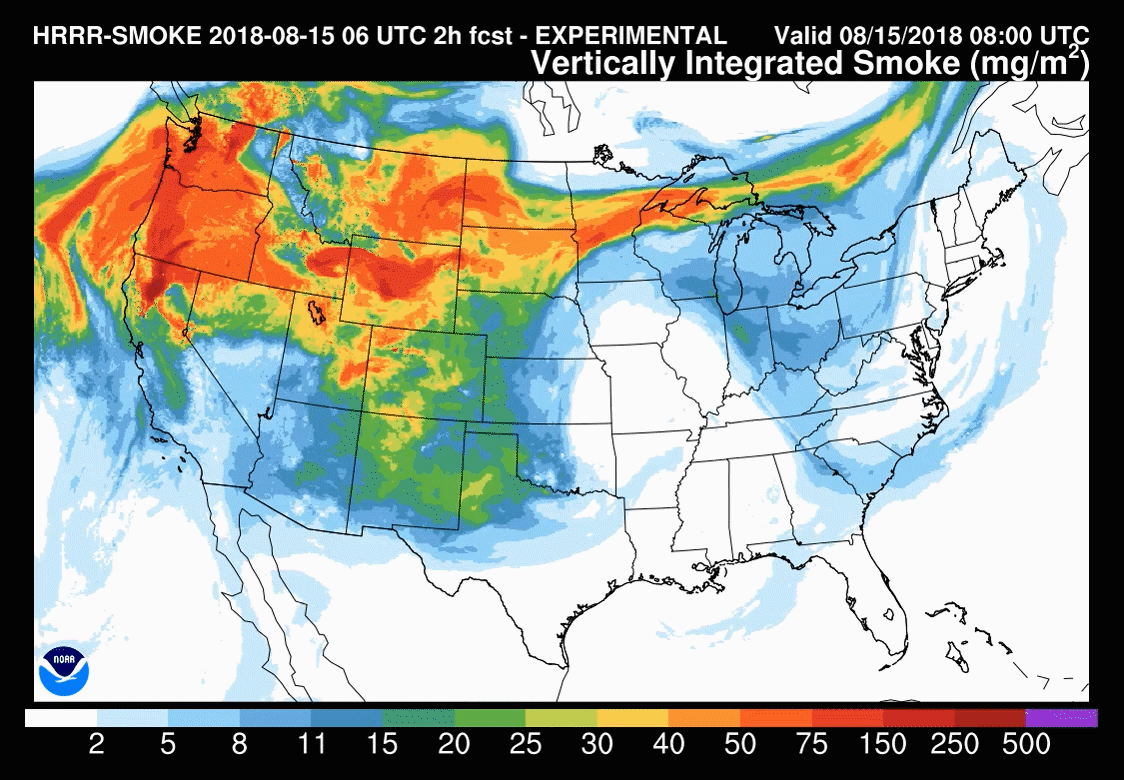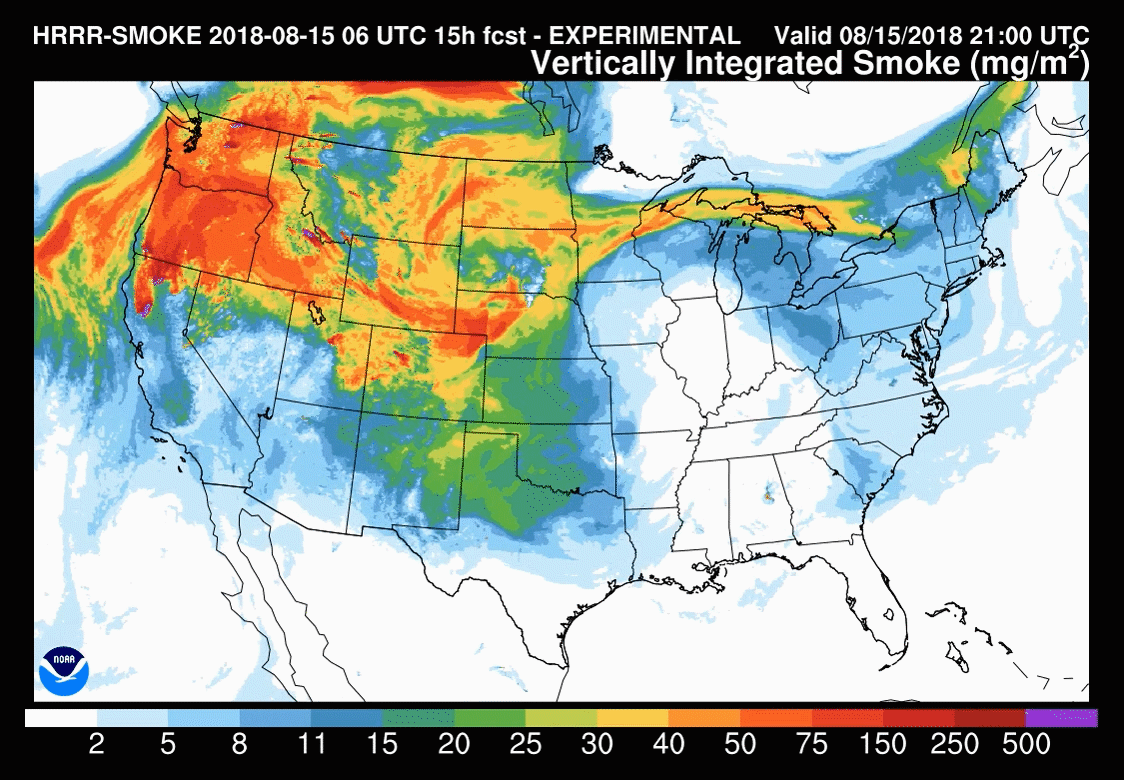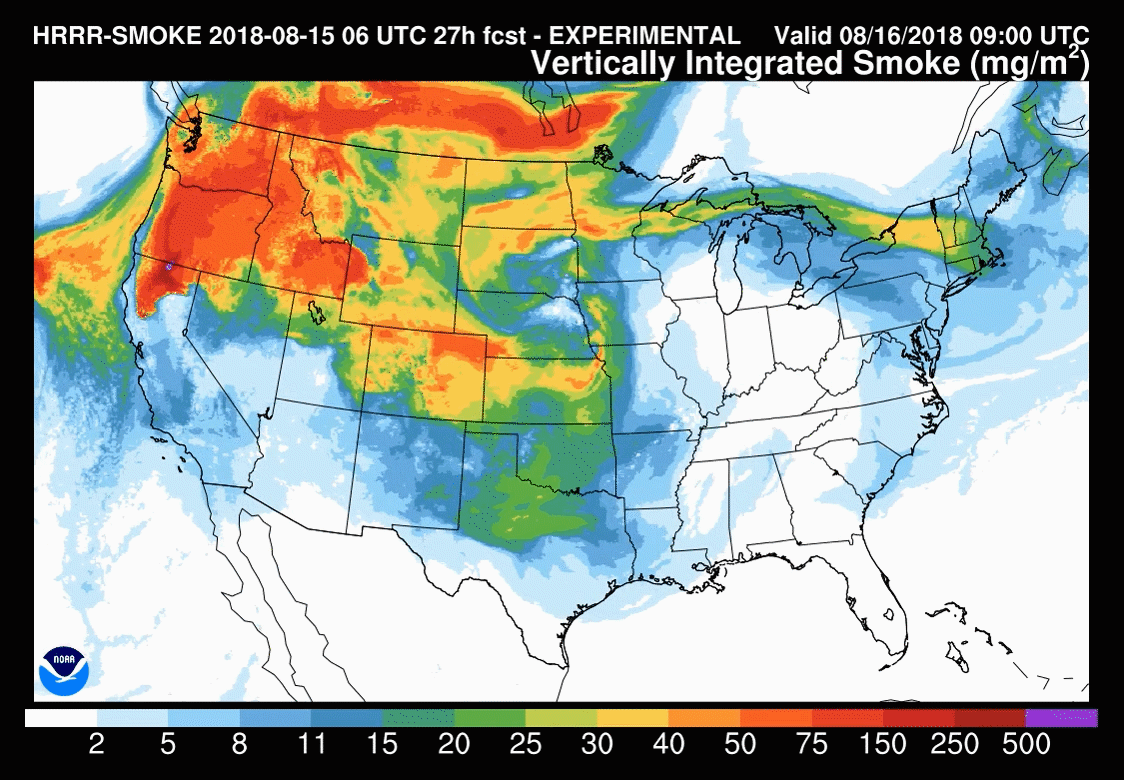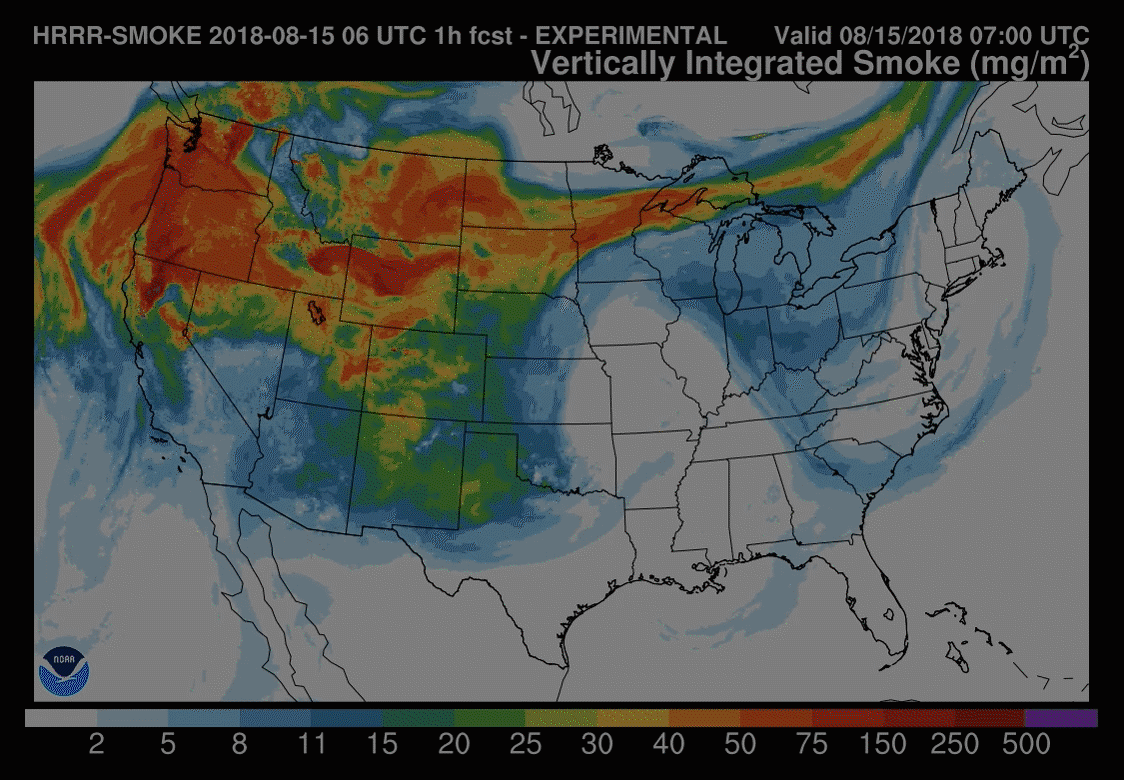
Models are increasingly used to forecast smoke-related air pollution issues. But smoke plume behaviour is notoriously difficult to predict, making these forecasts challenging. These tools are constantly under development with the end goal of being able to routinely forecast the precise location and severity of smoke up to several days in advance.
The US National Oceanic and Atmospheric Administration (NOAA) model – HRRR(high-resolution rapid refresh)-Smoke – is one such research effort. It was this model used to understand the November 2018 New York City haze. ‘Amtrak, our train system in the US, also used a forecast [from the NOAA model] to determine when they were going to shut down their train service in Northern California last summer, because of all the wildfires causing air pollution problems,’ says Christine Wiedinmyer, an atmospheric modeller at the Cooperative Institute for Research in Environmental Sciences (CIRES), a partnership between NOAA and University of Colorado Boulder.
Other efforts to simulate fire smoke and downwind impacts are being developed at Nasa, the US Forest Service and the EU’s Copernicus Atmosphere Monitoring Service. ‘Many models use information from satellites to tell us about when and where fires are burning,’ explains Wiedinmyer. Some of the models consider the amount of energy released when the satellite goes over, the type and density of the vegetation burning and how dry the fuel is. Likely emissions are then calculated and combined with weather forecasting information to determine where they are going to go and how they might behave in the atmosphere. ‘We push those pollutants around and react them, to figure out where the pollution is going to go and what impacts it might have,’ Wiedinmyer says.
Pulling together
Laboratory and field observations are vital for building, improving and testing models. And for puzzles as complex as smoke, huge collaborative research efforts can make real life data easier to understand. It’s much simpler to pick out what’s going on if multiple teams, armed with multiple instruments, measure multiple different chemical species in the same environment simultaneously.
‘If you have a complex chemical mechanism, and you see one of the species in this mechanism increase, and there’s five different reasons why it could have increased, it’s good to have other instruments [looking at other chemical species] that can help constrain the overall picture of the chemistry,’ explains Yokelson.
The summer of 2018 saw the largest, most comprehensive effort yet to study the chemistry of wildfire smoke. The We-Can (western wildfire experiment for cloud chemistry, aerosol absorption and nitrogen) project, funded by the US National Science Foundation (NSF), spent six weeks flying through smoke plumes from western US wildfires. Summer 2019 will see an even larger project take to the skies: the finale of the joint NOAA and Nasa effort Firex-AQ (fire influence on regional to global environments experiment and air quality).
Both field projects combine satellite, flying laboratory (aeroplanes full of analytical instruments) and ground-based measurements.
Yes We-Can
The main goal of We-Can is to systematically characterise emissions from western US wildfire plumes and observe how these gases and particles change during their first few hours in the atmosphere. ‘We wanted to look at the chemical evolution,’ says Delphine Farmer, an atmospheric chemist and We-Can participant from Colorado State University.
‘The wildfire plume came up and we flew into it as close as safe and legal to do so. And then we did passes in and out of the smoke for several kilometres as it moved downwind,’ she explains. ‘So it’s not exactly the same piece of smoke that we were measuring, but its close enough that we could follow the chemistry and physical evolution of the smoke with time.’ Using a high-resolution time-of-flight aerosol mass spectrometer, Farmer’s team was able to detect significant oxidation of organic particles in the smoke over time.
Much of the chemistry that happens in that time span is very, very rapid
This instrument was one of over 25 real-time analytical tools onboard the NSF/NCAR Hercules C-130 research aircraft during We-Can. These were crewed by scientists from 12 different research groups, from five universities and the National Centre for Atmospheric Research (NCAR), explains Emily Fischer, We-Can lead and Farmer’s Colorado State University colleague. Fischer’s group was charged with taking onboard ammonia measurements.
Atmospheric and cloud water samples were also collected for detailed analysis on the ground. The scientists and their instruments flew for over 130 hours, studying over 20 wildfires in detail and tracking smoke downwind for up to five hours. ‘Much of the chemistry that happens in that time span is very, very rapid,’ says Fischer.
A second, smaller plane – the University of Wyoming King Air – also participated. It flew a remote-sensing payload under the smoke, taking column measurements for a handful of gases. Yokelson helped coordinate the ground-based measurements, which featured numerous instruments in two mobile laboratories and in his lab at the University of Montana in Missoula, around 300 miles (480km) downwind from the Boise, Idaho, airport being used for the We-Can campaign.
Fire in the lab

Firex-AQ will bring together more than 30 research groups from four federal agencies and over 20 universities and companies. This summer’s air campaign is the finale of a multi-year project to explore the chemistry and fate of trace gases and particles in smoke from wildfires, prescribed fires (those purposely lit for hazard management) and agricultural burning.
The first field campaign for this project took place on the ground in 2016, inside the US Forest Service’s Missoula Fire Sciences Laboratory. Primary emissions from fires with various fuel types were studied in great detail in this controlled laboratory environment. ‘We took fuels from the western US and separated them out into individual components, like the duff at the bottom, the branches and so on,’ explains Carsten Warneke, a CIRES scientist who works at NOAA. They then burnt them separately and measured the emissions. ‘We also combined them to make as realistic a fire as we could in the lab.’
‘We analysed more than 90% of the smoke emission mass in great detail,’ he adds. The impact of fire temperature on emissions was also measured. ‘One goal [for the summer] is to see how well the laboratory study results compare to the real world,’ says Barry Lefer from the Washington DC-based Nasa earth science division.
Firex-AQ
Four aeroplanes, an uncrewed aerial vehicle and a handful of satellites are scheduled to take part in Firex-AQ this summer. ‘The first half of the project is based out of Boise, Idaho, and we’ll be focusing on western wildfires. Then we’ll move to Salina, Kansas, for the last three weeks and focus on agricultural fires that are smaller, shorter in duration,’ Lefer explains.
Fires are so intense they make their own local weather
The workhorse, with 150 flight hours scheduled and around 35 analytical instruments onboard, is Nasa’s DC-8 airborne science laboratory. ‘If you think about any chemical or an aerosol property that you might expect from fires, we can measure that. We got all the equipment on the plane that I had on my original wish list,’ says Warneke. His NOAA research group are taking a time-of-flight chemical ionisation mass spectrometer that uses hydronium ions to measure acetonitrile, speciated hydrocarbons and oxygenated volatile organic compounds. Jimenez’s group will be using a time-of-flight aerosol mass spectrometer to measure particle composition on the submicron scale.
A further 10 or so instruments on board a smaller NOAA Twin Otter aircraft will take additional measurements. And a second NOAA Twin Otter will look at how fires impact local wind. ‘Fires are so intense they make their own local weather sometimes which can feed the flames,’ Warneke says. Better understanding this phenomenon would help firefighting efforts.
The first Twin Otter will also collect night time measurements. ‘Fires burn really hot during the day meaning that the emissions are often lofted up high. But at night, when the fires start to die down, the smoke fills up the valleys, and that’s when you get the most health exposure from PM2.5,’ explains Warneke. ‘The DC-8 can’t fly low enough at night to get into the smoke.’ An unmanned aerial vehicle can fly even lower and while it carries a much smaller scientific payload, it will collect night time measurements that the Twin Otter cannot.
One Firex-AQ objective is to assess how satellites can better assist wildfire emission modelling and forecasting. Field campaigns allow us to ‘improve our understanding of what the satellites are really measuring’, explains Lefer. The high-altitude Nasa ER-2 aircraft will act as a satellite simulator flying high over fires carrying the same instruments currently on satellites, as well as next-generation analytical satellite tools.
The Firex-AQ campaign plans to track the smoke and its chemical evolution, for hours to days downwind. Ground-based measurements will be taken from two mobile laboratories and five fixed monitoring stations, including at Yokelson’s lab in Missoula. ‘We’re trying to look at the science around smoke as holistically as we can,’ says Warneke.
Influencing policy down under and beyond
The increasing frequency and severity of wildfires in recent decades is far from a US-only phenomenon. In 2009, Australia’s worst-ever wildfire event saw about 400 fires start during an extended drought. By the time they were extinguished, 173 people were dead and more than 2000 homes destroyed.
These Black Saturday fires led to a policy change – more prescribed fires to reduce fuel load in the environment and therefore the risk of larger, harder-to-control wildfires later. According to Clare Murphy, an atmospheric chemist at the University of Wollongong in Australia, this has caused unintended air quality issues. ‘The problem is that the conditions that are perfect to light up the hazard-reduction fires, and for them not to get out of control, so cold and low winds, are exactly the conditions that then cause temperature inversion and the smoke to sink and get trapped in basins overnight,’ she explains.
Murphy believes that more consideration should be given to smoke impact when planning prescribed fires in Australia. It is already common to run a smoke forecast in the US before prescribed fires. ‘I think that that’s almost within our grasp in Australia,’ says Murphy. The Australian national science research agency, CSIRO (Commonwealth Scientific and Industrial Research Organisation), has developed a model to assist with this. ‘This air quality model could be used as part of the decision process, to check if the wind direction’s going to change or to look at when the best window is,’ she says.
Peat fires are very different from vegetation fires
In the tropics, some atmospheric scientists have been investigating the air pollution related to changes in policy regarding using fire for land clearance. Since 2004, Brazil has significantly reduced Amazonian deforestation and therefore the associated deforestation fires. ‘We were interested in whether that decline in deforestation rate was also improving air quality,’ explains Dominick Spracklen, atmospheric modeller at University of Leeds, UK. He used a model and particle measurements from satellite and ground-based sensors to estimate that the resulting reduction in particulate matter is preventing up to 1700 premature adult deaths annually across South America.
Smoke from fires southeast Asia, such as the 2015 fires that poisoned the air for weeks, are also of interest to Spracklen. Laws to prevent the use of fires to clear forests for palm oil plantations are rarely enforced. Forest in this region grows on peat, which produces more smoke than other fuels. ‘Peat fires are very different from vegetation fires. There’s a lot more smouldering combustion and in dry conditions fires can burn deep into the peat. That is a double whammy in terms of the amount of particulates released from the fire,’ Spracklen says. ‘Quantifying the health impact should raise the issue up the political agenda in the region.’
Home fires still burning?
Peat was an issue during the three-week-long Saddleworth Moor fire near Manchester, UK, in summer 2018. During that fire, ground-based instruments including those in Coe’s city centre university labs – about 20 miles downwind of the fire – measured very high particulate matter levels. ‘If you were living within a couple of miles of that fire, the concentrations would have been really quite severe,’ Coe explains.

Particle composition is a particular concern in northern UK peat fires. ‘Because of Britain’s industrial past, there’s industrial material that has been deposited over the course of a hundred years or more across that region that is remobilised during peat fires,’ says Coe. ‘Some of the more volatile components, for example, lead and mercury, are re-volatilised into the gas phase and then re-condensed as fine particles. This is the same as the lead volatilisation that was reported from the fire in Notre Dame.’
To date, however, relatively few studies have looked at either the composition or the potential health impacts of UK wildfire smoke. This makes evidence-based policy decisions, such as the increased use of prescribed fires that have been called for by some, hard to make.
UK wildfire measurements largely come from the 300 or so ground-based air quality monitoring sites operated constantly for regulatory reasons. For large fires these tend to be supplemented by measurements using instruments at university labs and also those taken onboard the UK’s research aircraft – the Facility for Airborne Atmospheric Measurements. When studying wildfire smoke ‘there is a variety of instruments on board – those described as core for the more standard measurements as well as those for more exotic parameters’ that are supplied and operated by university research groups, explains Jim McQuaid, an atmospheric scientist at the University of Leeds. ‘Flight data can then be used to evaluate whether models, which try to replicate the fire emissions, are doing this accurately,’ says McQuaid’s PhD student, Ailish Graham. ‘This can then inform us of how many people were exposed to the fire pollutants.’ She is also using data from the ground-based regulatory sites during wildfires to model their health impacts for the UK population.
It’s important to note that the chemistry of wildfire smoke isn’t just important from an air quality perspective. Smoke composition and evolution also determines its impact on both the weather and climate. Many of the researchers above are also considering these, as the world learns to adapt to increasing numbers and size of wildfires. Scientists are also unearthing the reasons for the increased length and severity of wildfire season. The obvious answer, increasing global temperatures, is only part of the story.
Nina Notman is a science writer based in Salisbury, UK













No comments yet