From iron lungs to smartphone-controlled insulin pumps, Clare Sansom looks at the efforts to create artificial organs
About 10% of the NHS budget is spent on treating just one disease: diabetes. About 5 million Britons suffer from this disease, which for most patients is easily treated with insulin injections or, in the case of many with the commoner, more insidious type 2 disease, dietary restrictions and drugs. However, many people with diabetes still find it difficult to control their blood sugar levels to within the normal range, and much of that burgeoning health budget is spent on complications arising from this. In contrast, people with healthy insulin metabolism have exquisite control of their blood glucose levels. But what if this complex behaviour could be mimicked by a machine: an artificial pancreas? One of the most promising developments in this field is taking place at the University of Cambridge, UK, where a team led by Roman Hovorka is trialling a small, portable electromechanical device in diverse patient populations.
It is just over a century since the Canadian doctor Frederick Banting first injected insulin purified from the pancreas of cows and pigs into children dying from type 1 diabetes and rapidly restored them to health. The treatment soon entered mainstream clinical use, and Banting, who in today’s environment might have become a billionaire, sold his original patent to the University of Toronto for $1, memorably saying ‘Insulin does not belong to me; it belongs to the world.’ It is likely that some of the developments in treating insulin-dependent diabetes that occurred throughout the last century – replacing animal insulin with the engineered human protein, understanding that protein’s structure and mechanism, developing pancreatic islet cell transplants and insulin pumps – might not have taken place so rapidly without this generous act.
Even today, however, people with diabetes who are dependent on insulin have to second-guess how much they need before each meal, and they spend too long with suboptimal blood glucose levels, causing unpleasant side effects and building up health problems for later in life. Any system that can accurately be described as an artificial pancreas would have to mimic the ability of the natural organ to supply the exact amount of insulin that a patient needs to maintain a healthy blood glucose level, as near as possible to real time.
Portable pancreas
Scientists in Cambridge and elsewhere have been working with this principle for many years; Janet Allen, a senior paediatric nurse in Hovorka’s team, remembers some of the earlier devices developed there. The first, dating from the 1970s – long before Allen arrived – was the size of a filing cabinet and, she understands, rarely used. In contrast, the current version consists of three parts, the largest of which is a mobile phone.
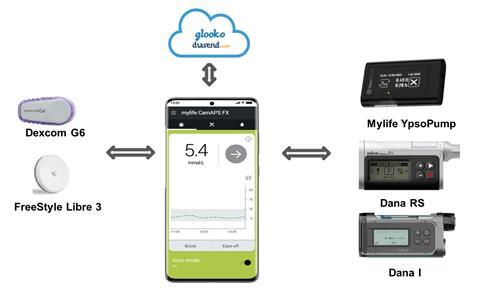
This device is still not, strictly speaking, a complete artificial pancreas as it does not reproduce all the functions of that organ; it is more precisely considered an automated, closed-loop insulin delivery system. It is built from two simple off-the-shelf pieces of kit – a glucose monitor and a phone-sized insulin pump – connected wirelessly to each other and to an app running an algorithm developed by Hovorka. This monitors glucose concentrations, automatically signalling the subcutaneous delivery of exactly the right amount of insulin so there is no longer a need for patients to test regularly. ‘The devices communicate to each other wirelessly using Bluetooth,’ says Rama Lakshman, a clinical research fellow working with the group. ‘If Bluetooth goes down there will be no link to the sensor, so baseline insulin requirements are programmed into the system to keep each patient going until a network is restored.’
The sensor can detect a patient’s glucose level and send a signal to the pump to deliver glucose subcutaneously every few minutes, but this is still not as fast or as sensitive as the pancreas it replaces. ‘We therefore need to be able to use a form of insulin that works very quickly,’ adds Lakshman. The most suitable insulins will have sequence alterations that prevent them from forming stable hexamers in solution, so they are rapidly absorbed into the bloodstream.
This system is available on the NHS, but only to patients with type 1 diabetes who meet specific criteria. In clinical trials, its use is being expanded into further patient populations, including patients whose type 2 diabetes has led to kidney failure and other severe complications, and young children. ‘It has been tested in children as young as two, who can run around and play normally while using it,’ says Allen. And dual hormone devices that release glucagon as well as insulin are being developed, adding a second function of the natural organ and taking us a further step towards a true artificial pancreas.
From iron lungs to Covid-19
A little machine like this is probably not the first thing that comes to mind when you hear the term artificial organ, however. You are more likely to think of an implanted electronic device, and perhaps of the idea, still restricted to science fiction, of the bionic man. But extracorporeal devices, worn outside the body like this one, have major advantages. They are more accessible than implanted ones, and therefore easier to repair or replace.
The first such machine to have a claim on the term artificial organ is probably the iron lung, a mechanical ventilator that kept paralysed polio victims alive, sometimes for years, in the early part of the 20th century. However, these patients remained paralysed and immobilised inside the machines, with very low quality of life. The first insulin pumps, too, were very large. The trend throughout the decades has been towards smaller, smarter devices that replicate necessary physiological function and are small enough to give patients a relatively normal life. This helps the patients not only psychologically, but physically, as Joseph Zwischenberger of the University of Kentucky, US and president of the American Society for Artificial Internal Organs in 2022 explains. ‘We evolved to be hunter-gatherers, that is, to move around all the time. Bed rest, and perhaps particularly bed rest attached to a machine, leads to muscle wasting and other physiological difficulties.’
During the first wave of Covid, over half the sickest patients put on ECMO walked out of the door of the ICU
Zwischenberger, a cardiovascular surgeon, has dedicated much of a distinguished career to developing the extracorporeal membrane oxidation (ECMO) machines that support the functions of the heart and lungs for short periods and that became familiar to many at the height of the Covid-19 pandemic. He dates his interest in the mechanics of human physiology to an adolescence spent tinkering with vintage cars. ‘An automobile from the 1930s is simple enough for you to learn how the parts work separately and how they interconnect,’ he says. ‘There are clear analogies with the physiology of the human body, with, for example, the heart as its pump and the lungs as its gas exchange mechanism.’
He began work on ECMO in Kentucky in the early 1980s when, with his mentor Robert Bartlett, he developed an effective machine to support heart and lung function, initially for new-born babies with severe lung disease. Later, the team invented the first double-lumen cannula with two tubes for gas exchange. Patients were able to walk around while attached to these machines, which were used as a bridge to lung transplants, but they really came into their own during two 21st century pandemics: H1N1 influenza in 2009 and then Covid-19. ‘During the first wave of Covid, over half the sickest patients put on ECMO walked out of the door of the ICU,’ he remembers. ‘The only problem we had was with finding enough machines, so we rationed their use to patients under 55, which would have excluded me.’ Since then, vaccination and mutation has changed the nature of the disease and very few Covid patients now require such intensive heart and lung support.
Donor or device?
Transplantation is considered the gold standard for treating patients with kidney failure, but there is one important problem: a severe shortage of donor kidneys. There are currently three million US kidney patients who would benefit from a transplant, and less than 100,000 kidney transplants performed there each year. ‘In this country, only one in five patients on the kidney transplant list ever gets a transplant… and there are delays in getting on the list in the first place’ explains Shuvo Roy, a bioengineer at the University of California San Francisco, US. Similar statistics can be quoted for many other countries, and this worldwide problem is likely to get worse. Obesity, diabetes and hypertension – all important drivers of kidney failure – are increasing in prevalence, and the number of donor kidneys is no more than stable. Patients waiting for transplants fall back on dialysis, which is unpleasant for the patient and costly for the health system; much of its cost arises from frequent complications including infections and blood clots, and from hospital stays.
The pig is the most appropriate mammal to use because it is closest to humans physiologically
Roy is the director of the Kidney Project, an award-winning US-based project to create a ‘small, surgically implanted, free-standing bioartificial kidney’ to treat kidney failure, particularly, but not only, in patients waiting for transplants. His team have developed a device that is, essentially, an implantable miniaturisation of a machine used to treat acute kidney failure in the ICU. This original extracorporeal artificial kidney, the Renal Assist Device, is impracticable for use outside critical care because of its high cost and large size. Both devices have the same two main components: a haemofilter that filters blood efficiently at pressures no higher than those in the capillaries, and a cell bioreactor of renal tubule cells. This can reabsorb salt and water but not toxins and replicate some of the other biochemical functions of a kidney, including the production of vitamin D. ‘We have developed a small-scale, functional prototype of this system that can produce urine when implanted in a pig,’ explains Roy. ‘The pig is the most appropriate mammal to use because it is closest to humans physiologically, and the size of the device is also similar to that needed for human patients.’
The team is already talking to the FDA about taking their device into initial clinical trials. One necessary improvement will be to ensure that it can remain longer in the body without problems. The current prototype has worked continuously for 3–7 days when implanted in a pig, but in clinical use it will need to work for at least a month between replacements. ‘We need to find a coating for the device that further enhances its biocompatibility, so that the cells in the bioreactor stay alive for longer,’ adds Roy. An ideal implanted artificial kidney would last much longer than this – essentially permanently, like a heart pacemaker – but this is still a long way off.
The risks arising from this device’s contact with an animal or human bloodstream are common to all implanted artificial organs that involve a non-biological component: blood clotting, inflammation and an immune response. The first of these can be reduced if devices are made from animal tissue or stem cells, and the others are reduced or changed if the organ concerned is involved in the immune system. Work to develop an artificial thymus, for instance, involves an entirely different set of challenges.
The neglected thymus
Much less is understood about the function and mechanism of the thymus than of most other organs; in fact, it remained almost unknown until the 1960s. It is a lobed structure that sits in the upper chest, in front of the heart, and it is the site where one class of white blood cells – the eponymous T cells – develop. Immature T cells, known as thymocytes, learn there to distinguish between self and non-self proteins, and to mount an immune reaction to those not recognised as self. A healthy immune system includes an enormous variety of T cells adapted to respond to different non-self proteins, and some others, known as regulatory T cells, that suppress any immune response to self ones. Thus, the T cells, and the thymus in which they are formed, are essential for maintaining the immune system in balance, preventing the over-response that causes auto-immune disease while maintaining the ability to respond efficiently to attack by pathogens.
In some situations, an artificial thymus can be less risky than a transplant
Paola Bonfanti, who leads a group researching thymus biology and regenerative medicine at the Crick Institute in London, UK, describes the thymus as a ‘neglected organ’, and there are two connected reasons for this. As an individual’s T-cell repertoire must develop by early childhood, the thymus is most active before and soon after birth and then begins to degrade, with thymocytes replaced by fat cells. In adulthood this vestigial organ can be removed, for example by a cardiac surgeon needing easy access to the heart, with little ill effect.
In contrast, the thymus is essential in early life. Children born with mutations involving genes on part of chromosome 22, such as DiGeorge’s syndrome, often have no functional thymus. These children are very severely immunosuppressed; if the thymus function cannot be replaced, they must remain in sterile environments as so-called bubble children. ‘In one way these kids are ideal transplant patients, as they have no T cells to attack the non-self donor thymus,’ explains Bonfanti. ‘However, a donor organ will always contain some of the donor’s T cells, and the boot is therefore on the other foot: the donor T cells will attack the recipient’s tissue, causing a type of graft-versus-host disease.’ In this situation, an artificial thymus can be less risky than a transplant. Bonfanti and her colleagues have generated ‘clean’ thymus tissue that is clear of T cells from stem cells and are working with Great Ormond Street Children’s Hospital in London to implant it into severely immunodeficient children. ‘This therapy could also be useful for other patients with acquired immune deficiencies,’ concludes Bonfanti.
The term ‘artificial organ’ covers a wide variety of applications, as these four case studies show: implanted or extracorporeal; electromechanical or stem-cell based. There are many others. Scott Nyberg of the Mayo Clinic in the US state of Minnesota is setting up a company to develop a ‘bioartificial liver’ as an alternative to liver transplant, and synthetic red blood cells are being developed to carry drugs as well as oxygen round the body. We may still be far from the bionic man of science fiction, but millions more people will come to benefit from artificial organs already in the clinic and those in development.
Clare Sansom is a science writer based in Cambridge, UK
















No comments yet