Polymers that grow like trees have been around for nearly three decades. Now they are on the verge of realising their potential, as Michael Gross reports
Polymers that grow like trees have been around for nearly three decades. Now they are on the verge of realising their potential, as Michael Gross reports
After years of fundamental research, the tree-like polymers known as dendrimers are now ripe for commercial development, particularly in the field of drug delivery, where their spacious cavities are crying out to be used.

The idea of using branched monomers to produce a tree-like polymer (or oligomer), blossomed at the University of Bonn, Germany, where Fritz V?gtle’s group designed and synthesised the first such ’cascade molecules’ in 1978.
However, it was only in the 1980s - when fractals such as the Mandelbrot Set became fashionable - that other scientists also took an interest in fractal molecules. The more widely publicised work of Donald Tomalia (then at Dow Chemicals) kicked off a new research field, which has grown steadily over the past two decades and is now breaking into the R&D mainstream. Tomalia introduced the name Starburst dendrimers, which has become a trademark for commercially available dendrimers.
Inside out versus outside in
There are several fundamentally different ways of making dendrimers. The first approach that everybody would probably intuitively think of when seeing such a structure is to grow it like a tree from the root towards the tips of the fresh shoots. This is now known as the divergent approach. It starts from a core molecule with two or more functional groups, to which the branches are added stepwise, one layer at a time.
In order to avoid uncontrolled irregular growth in one direction, chemists typically design the synthesis such that each layer requires two steps that are separated. For example, they might attach Y-shaped branching monomers to the core, but they would make sure that the outer tips of the Y units do not yet carry the functional group to which the next generation of Ys can be attached. Rather, they would make sure that the current layer (or ’generation’) of monomers is complete, then carry out a reaction that activates the tips such that the next layer can be added.
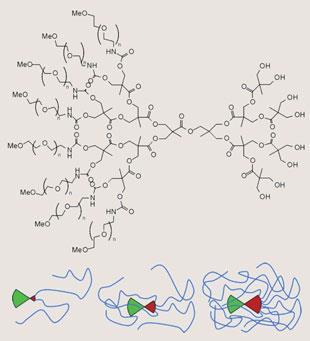
Even with this precaution, there are limits to the homogeneity of such molecular trees. After several generations, space will be in short supply, as the number of monomers to be accommodated at the surface of a roughly spherical molecule grows exponentially, while the surface area of the growing sphere scales only with the square of the generation number. Therefore, there is the risk that reactions will be incomplete, and the resulting dendrimers will be heterogeneous in their molecular composition.
This is the main reason why some researchers have switched to the alternative route, known as convergent synthesis, where the branches are assembled from the outside in and then attached to the core in the last step. While this doesn’t exclude the possibility that incomplete variants might occur, purifying the desired product becomes much easier for a very simple reason. If a divergent synthesis fails to run to completion, there will be only little twigs missing at the periphery, and the incomplete molecules will be difficult to separate from the desired perfect dendrimer. In convergent synthesis, a whole big branch will be missing, resulting in a substantial difference in molecular weight and physical properties, allowing easy separation.
Traditional dendrimers are produced by either of these strategies using organic chemistry to tie the branches up with covalent bonds. However, in recent years there have also been efforts to produce self-assembling dendrimers from non-covalent interactions (Chemistry World, April 2005, p14). Furthermore, dendritic branches have been studied on their own, as low molecular weight compounds (dendrons) and attached to long-chain polymers (hyperbranched polymers), opening up the size range of dendritic molecules.
Drug trafficking
As surface crowding increases with the number of generations, typical fifth generation dendrimers have a very dense, spherical surface covering spacious cavities in the layers closer to the core. The idea that these might serve as vessels to shuttle drugs or genes into cells was mooted quite early on. However, it took some time to evaluate the toxicity of dendrimers and find out which of the potentially infinite number of possible molecular trees were best suited for a job as molecular transporter in the body.
As a general rule of thumb, dendrimers with anionic (eg carboxy) end groups are more suitable for medical applications than their cationic counterparts, which are more toxic. Several studies suggest that certain types of dendrimers can serve as shuttles for drugs that are known to be effective in vitro but fail to survive in the body if administered on their own.
Antony D’Emanuele from the University of Central Lancashire, UK, and his team have used dendrimers to create prodrugs, which are administered in an inactive form but metabolised in vivo into the active compound. They did this by covalently coupling the model drug propranolol to a surface modified version of the commercially available polyamidoamine (PAMAM) third generation (G3) dendrimer. The dendrimer successfully transports the drug into living cells, claim the researchers. More recently, the same group has expanded this work using a range of different dendrimers and imaging methods to show that the drug is delivered inside cells. They have optimised the pH-dependent stability of the covalent bond between the dendrimer and the drug such that it becomes cleavable once the prodrug has entered the cell.
Jean Fr?chet and Francis Szoka from the University of California at Berkeley and San Francisco, respectively, have developed a different approach based on molecules they have dubbed ’bow-tie dendrimers’. Following the convergent synthesis strategy, these consist of two different molecular trees attached to a core molecule. The researchers use one side of the bow tie to optimise the solubility of the construct, and the other to create suitable binding sites for a drug to be carried.
Recently, the Californian groups have shown that mice with solid tumours that are normally resistant to the drug doxorubicin can be cured of their cancers by a single dose of the drug coupled to such a bow-tie construct.
In a separate advance that may prove useful for the development of new cancer drugs, Jean-Pierre Majoral and his colleagues at the Centre National de Recherche Scientifique (CNRS) in Toulouse, France, have shown that phosphonate groups coupled to dendrimers can induce a rapid multiplication of human natural killer cells, which help the immune system to combat cancer.
Dendritic drugs
The medical applications of dendrimers extend beyond targeting drugs to cancer cells. The most advanced dendrimer-based drug is VivaGel, a vaginal antiviral gel designed to prevent sexually transmitted infections, including HIV and genital herpes. In this formulation, which has been developed by the company Starpharma in Melbourne, Australia, the dendrimer is not the carrier but the active ingredient. With its branched structure it binds viral surface proteins and prevents them from attaching themselves to human cells.
Phase I trials have shown that the drug is safe and well-tolerated. Further trials to test its efficacy against genital herpes and HIV are now underway in Australia, the US and Kenya, backed by a major grant from the US National Institutes of Health (NIH). There are also investigations into the possibility of using VivaGel as a condom coating, and as a contraceptive.
Meanwhile, Sunil Shaunak’s group at Imperial College London has developed dendrimer glucosamine conjugates to target molecules involved in inflammatory response and scarring. They hope that these constructs will become useful drugs against bacterial sepsis and scarring after eye surgery.
Good gels
Samuel Stupp’s work to use dendrimers for bone fixing and wound healing gets V?gtle’s vote as ’the most spectacular’ recent result in the medical application of the polymers. Stupp’s group at the Northwestern University in Evanston, Illinois, US, has developed dendritic gelators - dendrimers that self-assemble in the presence of physiological fluids to form nanofibres and gels. The nanofibres are capable of signalling to integrin receptors in mammalian cells. According to Stupp, they exhibit a very high degree of bioactivity, and can serve as a great artificial extracellular matrix in regenerative medicine. What is more, they can be customised for specific types of cells.
’Preliminary results suggest that they can be used to fill in breaks and gaps in bones and cartilage, and are efficient in curing joint malfunctions in mice,’ says Stupp. ’They cure mice of spinal cord injury by stimulating the regeneration of axons: the nanofibres do this by displaying biological signals that can bind to receptors on the membranes of neural cells.’ However, Stupp points out that the experiments with mice were done with linear analogues of the dendritic molecules. ’Nonetheless we expect the dendritic forms (based on research so far) to be even more bioactive by facilitating access of the signals to receptors,’ he adds.
Using similar but chemically different nanofibres, Stupp’s team has also developed a nanofibre scaffold which, when combined with the biopolymer heparin, promotes blood vessel growth and could be important in wound healing.
Creative catalysis
To the chemist, a dendrimer’s fractal structure and surface immediately raises the question of whether it can be made useful as a catalyst. Small enough to remain in solution, but large enough to be filtered or sedimented out of it when necessary, dendrimers could in theory combine the best of the mostly separate worlds of homogeneous and heterogeneous catalysis.
According to V?gtle, there have been partial successes in this field ’but the big breakthrough hasn’t materialised yet’. He blames this disappointment on the fact that dendrimer researchers are spoilt for choice. ’Dendrimers offer an infinity of possibilities for changing the size, shape and softness of their design, so they should be ideal. But the disadvantage is that, exactly because there are so many possibilities, there is a lot of trial and error involved, which costs time and money,’ he tells Chemistry World.
A recent example of such ’partial successes’ is the work of Jean-Pierre Majoral and Marc Taillefer and their groups at the French CNRS research centres in Toulouse and Montpellier, respectively. These researchers used dendrimer-bound imino-pyridine ligands for copper(i) catalysis of the coupling reaction between aryl or vinyl halides with nucleophiles such as phenols or pyrazole. They claim to have achieved efficient copper catalysis of C-N, C-O, and C-C bond formation ’under some of the mildest conditions ever reported’.
Majoral comments: ’A remarkably strong positive effect of dendrimers compared with that of monomeric species is observed in these experiments. Although some unique features of dendrimers in catalysis have already been reported (very high local catalyst concentration, advantages of both homogeneous and heterogeneous catalysis, easy catalyst recovery and reuse without significant loss of activity), such a dendritic enhancement is very rare in the field of organometallic chemistry, opening new perspectives for applications in life sciences and in the field of materials.’
Synthesis matters
Even if there is an impending goldrush of dendrimer applications in medicine and other fields, the fundamental research remains important, as there are still new aspects to explore and synthetic procedures that could be improved.
Dendrimer researcher J?rn Christensen from the University of Copenhagen, Denmark, stresses the importance of synthesis research. ’I think that the bottleneck at present is in the synthesis of dendrimers,’ he tells Chemistry World. ’For many applications in the biological field, dendrimers with a specific substitution pattern would be highly desirable. In my opinion, there is a need for more work on how to synthesise well-defined dendrimers in a simple manner.’
Among the researchers thinking along the same lines is Oleg Lukin at ETH Z?rich, Switzerland, who recently synthesised what he calls ’designer dendrimers’ with fine-tuned molecular architecture. Lukin’s approach involves including sulfonimide groups in every branching unit and using their natural chemical selectivity to guide synthesis towards the desired form and shape.
The name of the game
When researchers have succeeded in designing and synthesising such new molecules, there remains the tricky question of naming them. The traditional Iupac (International union of pure and applied chemistry) nomenclature requires identification of the longest chain, which becomes a pointless exercise if there are chains of equal length branching off in all directions.
Therefore, V?gtle recently developed a new comprehensive system of dendrimer nomenclature, based on an earlier effort of George Newkome, University of Akron, Ohio, US. While staying compatible with Iupac as far as possible, the system takes into account the special fractal geometry of dendrimers, much as the special nomenclatures for other groups of unusual molecules - such as catenanes or knots - made provisions for the idiosyncrasies of these groups.
With his naming system, V?gtle also managed to lead the field he initiated nearly 30 years ago back to its cascade molecule roots. At the core of any dendrimer name , analogous to the word ’catenane’ for chain-linked molecules, will be the word ’cascadane’.
The names of these clever molecules will little alter what is no doubt a promising future. Current successes in the development of dendrimer-based drugs, drug carriers, and materials suggest that we are going to hear a lot more about them in the near future.
Michael Gross is a science writer based in Oxford, UK.
The 5th International Dendrimer Symposium will take place in Toulouse, France, on 28 August-1 September 2007. The New Journal of Chemistry(RSC-CNRS) will publish advance papers from the conference in its July 2007 issue - see the NJC link.
Further Reading
- U Boaset al, Dendrimers in medicine and biotechnology, RSC Publishing, Cambridge, UK, 2006
- M Najlah et al, Int. J. Pharmaceut., 2006, 308, 175
- C C Lee et al, Proc. Natl. Acad. Sci. USA,2006, 103, 16649
- L Griffe et al, Angew. Chem. Int. Ed., 2007, 46, 2523
Companies
Starpharma, Australia, develops dendrimer nanotechnology products for pharmaceutical, life science and other applications.Its lead product, VivaGel, is a dendrimer-based formulation being developed as a vaginal microbicide. In October 2006, Starpharma acquired Dendritic Nanotechnologies, US. This company develops and provides advanced dendritic polymers. Its priostar dendrimers are for use in sectors ranging from cosmetics to speciality chemicals.
Dendritech, Michigan, US, is a speciality polymer company which focuses on developing dendritic, hyperbranched and tailor-made polymer products. It offers to produce large quantities of dendrimers, in particular ethylenediamine-core poly(amidoamine) (PAMAM) structures.
Polymer Factory, Sweden, provides unique dendrons and dendrimers built from 2,2-bis (methylol) propionic acid (bis-MPA) monomer. This can be used in a variety of applications ranging from drug-delivery systems to photonics. Polymer Factory was founded in 2005 in the fibre and polymer technology department at the Royal Institute of Technology in Stockholm, Sweden. The company has the exclusive rights to the marketing, production and sales of dendrimers based bis-MPA.
Frontier Scientific, Utah, US, sells so-called ’Newkome’ dendrimers and dendrons through collaboration with George Newkome at the University of Akron, Ohio, US. Initial offerings include five dendrons, the branched building-blocks for dendrimer synthesis, and four dendrimers. The company expects to expand its range of dendrons.
DSM, the Netherlands, sells its Astramol dendrimers for ’high-end markets such as diagnostics, solar cells, sensors and DNA transfer’.






No comments yet