Medical imaging now promises to take us to the molecular level, thanks to new, powerful MRI machines and clever contrast agents, as David Bradley finds out
Medical imaging now promises to take us to the molecular level, thanks to new, powerful MRI machines and clever contrast agents, as David Bradley finds out
If scanners are the hardware of medical imaging, then chemical contrast agents must surely be the software. As such, physicists are the hardware engineers, and chemists the software writers who integrate disease and imaging information to come up with answers.
Contrast agents for medical imaging and in particular magnetic resonance imaging (MRI) and positron emission tomography (PET) were introduced into clinical practice two decades ago, with 30-40 per cent of MRI scans using them. ’Contrast agents are exciting and very useful species for chemists as well as for physicists, biomedical engineers, biologists, and clinicians,’ says Willem Mulder of the radiology department at Mount Sinai Medical Center, New York, US. ’They are a bridge between all the different fields of research that aim to visualise [disease] processes in vivo.’
The infamous barium meal is perhaps the most well known chemical imaging agent, but other elements, including bismuth, caesium, and silver also make simple contrast agents. More sophisticated agents based on iodine have allowed x-ray imaging of soft tissues, such as the oesophagus, heart, and kidneys. Iodinated compounds, such as metrizamide and iopamidol, have also provided insights, but these agents are at best adequate for many tasks. Meanwhile, the radio-opacity of the lanthanides gadolinium and holmium gives high contrast and safer, shorter x-ray exposure times.
Digital processing via a charge-coupled device in the form of the CAT scan (computerised axial tomography), produces high resolution information and captures slice after slice of information to produce a three dimensional picture. Computers then extract the subtlest of data allowing x-rays to distinguish between different tissues depending on contrast agent absorption.
On target?
Version two contrast agents are now needed to cope with the growing demands of the hardware and the clinicians. New molecules that provide higher contrast and clearer images not only work at lower dose in more powerful scanners but are also safer. ’As contrast agents need to be administered in large quantities, typically two grams per patient,’ explains David Parker of the University of Durham, UK, ’any new system must be relatively cheap to produce and must show an order of magnitude better behaviour than current ones.’ This, he adds, is especially true at the higher field strengths (three Tesla) at which new MRI machines operate. With new agents coming online, another benefit is emerging: the ability to target specific organs, disease sites, and even individual cells in the body with higher selectivity than ever before.
To date, there are few novel targeted CT contrast agents. However, Jamey Weichert and colleagues at the University of Wisconsin-Madison, US, are making inroads. They are developing polyiodinated triglyceride compounds encapsulated in a microemulsion. These are proving useful in hepatobiliary (liver and bile) imaging and lymphography. According to Weichert, their approach distinguishes between healthy and tumour tissue in the liver. Conventional CT cannot make this distinction but Weichert’s contrast agents can selectively target liver cells and with microCT imaging provide a clear contrast between the two. One focus of such work is in cancer research involving mouse models of liver cancer. Recently, the team imaged tumours just a couple of millimetres in diameter that would have been invisible to conventional CT.
Magnetic resonance
It is in the field of MRI that contrast agents have come into their own. The technique is based on one of the analytical chemist’s favourite tools, nuclear magnetic resonance (NMR) spectroscopy. It is a great water observer, allowing body imaging to show regions dense with the ubiquitous solvent. MRI is usually carried out like a CT scan with a slice by slice view of an organ of interest added together by nifty computation to produce a three-dimensional image.
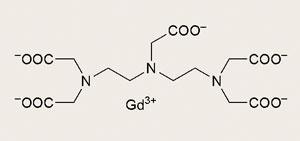
The clinical breakthrough came with German firm Schering’s gadolinium chelating agent which both carries the metal and masks its toxicity. Schering’s patent application for the archetypal compound, Gd-DTPA (diethylenetriaminepentaacetate) dimeglumine, was submitted in July 1981 by company researchers Hanns-Joachim Weinmann and Ulrich Speck and would become the natural successor to earlier contrast agents based on iodine. In 1984, Dennis Carr of the Hammersmith Hospital London, UK, and Wolfgang Sch?rner in Berlin, Germany, demonstrated the first images in humans.
As with x-ray imaging, MRI benefits from contrast enhancing agents. Specific agents have been developed for imaging the various organs and systems of the body. As such, there are gastrointestinal contrast agents that allow the radiographer to distinguish between the normal organs, the bowel, and intra-abdominal masses. These agents include perfluoro compounds, barium, and solutions of ferric ammonium citrate (Geritol). There are now tumour-specific agents, liver and biliary contrast agents, and contrast agents for the reticuloendothelial system of spleen, lymph nodes and liver.
Other contrast agents exploit the superparamagnetic properties of iron oxide nanoparticles, for example. These negative contrast agents appear very dark and have been used to image the liver, where normal liver tissue retains the agent and appears very dark, but abnormal areas, such as scars and tumours, are lighter.
These agents are not limited to intravenous administering; they can be taken by mouth to improve imaging of the gastrointestinal tract without interference from water.
Designer images
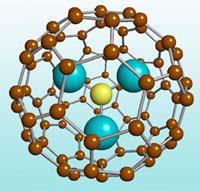
Aside from iron oxide nanoparticles, designer agents are emerging that exploit the unique properties of structures on this scale. Virginia, US-based start-up Luna nanoWorks, for instance, is now commercialising an outsized buckyball ([80]fullerene) that holds three Gd ions within. This nanoparticle can cope with the high-power MRI scanners where lesser Gd agents fall short.
As if imaging tissues at high resolution and even at the cellular level were not enough, Alan Jasanoff and colleagues at the Massachusetts Institute of Technology, US, are also using iron oxide particles for molecular imaging. In late 2006 they reported a new family of calcium indicators for MRI that combine iron oxide nanoparticles with the calcium-sensing protein calmodulin. Protein interactions that respond to calcium concentration make the iron particles aggregate and so contrast in these regions is raised enormously. The researchers have found one agent to be suitable for detecting elevated intracellular calcium levels. This, they explain, could assist molecular imaging of biological signalling processes in living specimens.
There are other examples of using paramagnetic particles to target blood vessels in tumours. Mulder and his US research team have reported how cell processes can be imaged using quantum dots with a paramagnetic coating. Angiogenesis (blood vessel growth) and the process of apoptosis, or programmed cell death - inextricably linked to development, growth and cancer - can be tracked using MRI and a quantum dot agent, they found.
They imaged angiogenesis using quantum dots labelled with peptides containing the motif arginine-glycine-aspartate (RGD). The RGD peptides interact with receptor sites that can initiate cell signalling processes. RGD peptides help cells to link to the extracellular matrix. If cells don’t link, they can undergo apoptosis.
Mulder and his team have also conjugated quantum dots to the cellular protein annexin A5 and coated it with a lipid. ’The lipid shell (micelle) is paramagnetic and provides the particle with paramagnetic properties,’ Mulder explains. These quantum dots can then home in on apoptotic cells and the paramagnetism provides an image of cell death almost as it happens.
A Gd move
Durham’s Parker and colleagues have worked for many years on contrast agents that use Gd to improve the image. Paramagnetic metal ions such as Gd produce strong magnetic resonance signals and became an early focus of attention for the development of contrast agents in the 1970s when MRI was first emerging from the laboratory and entering clinical practice.
There are many medical situations in which the widespread distribution of the contrast agent into the fluid space between cells confuses the image. For instance, an image may be required of renewed blood flow to a previously ischaemic organ or of the degree of blood vessel growth in a tumour. By attaching a high molecular mass chemical to the Gd-DTPA contrast agent it is possible to preclude its spread to the intercellular space and limit it to blood vessels only. Gd-DTPA can be attached to a protein, such as albumin, or a polysaccharide such as dextran. The albumin complex is useful in liver, spleen, myocardium, and brain imaging while the dextran complex adds the kidneys to this repertoire.
It is also possible to tag whole red blood cells with a contrast agent, such as isotope-51 of chromium(iii). This complex is also restricted to intravascular regions but allows the liver to be imaged without interference from diffusion into the kidneys.
Parker’s team recently published results on a new approach to Gd contrast agents based on surrounding the metal ion with a medium molecular weight glycoconjugate containing a dozen glucose or galactose units. This novel complex has the advantage of being effective at the new high fields available to MRI, because the bulky mass of the glycoconjugate raises the intrinsic relaxivity of the system - the ability to increase the relaxation rates of the surrounding water proton spins and give improved image contrast. Sugar complexes maintain water solubility and so ease excretion.
In October 2006, Parker and his colleagues at the North of England Cancer Research Campaign and Manchester Wolfson Imaging Centre, UK, were awarded a major interdisciplinary grant to explore novel contrast agents that can be used in MRI and related PET scanning systems. ’In the context of our own efforts to examine dual imaging agents for both MRI and PET, others have demonstrated proof of principle and our efforts relate to a common ligand strategy [for such agents],’ Parker says.
Nature’s agents
Gang Zheng, Ian Corbin, and co-workers at the University of Toronto, Canada, have turned to natural rather than synthetic structure to help them create novel agents. They have shown that paramagnetic LDL (low density lipoprotein) particles can be used to image tumours as well as normal tissues expressing the LDL receptor; one of the few examples of intracellular MRI. ’Lipoproteins are attractive nanocarriers for delivering imaging agents to cancer cells,’ Corbin tells Chemistry World. Such particles are endogenous and so biocompatible, biodegradable and non-immunogenic, he explains. ’One drawback of this approach is the narrow purview of lipoprotein receptor-positive cancer cells.’ To overcome this limitation the team has recently developed a simple yet effective way of redirecting lipoproteins from the native receptor in the body to alternative receptors or surface markers selectively expressed on cancer cells. ’Such advances will increase both the range and accuracy of cancer directed imaging agents,’ explains Corbin.
In contrast, Zahi Fayad and Edward Fisher, at Mount Sinai School of Medicine, US, have used high-density lipoproteins (HDLs) loaded with Gd to image the potentially dangerous plaques that line coronary arteries in atherosclerosis. These plaques can break apart at any stage in a person’s life, whether they are fit or not, leading to cardiac arrest and death. ’We want to be able to pinpoint which plaques are active,’ explains Fayad, ’because cardiologists lack a biochemical marker that can reveal this.’ He and his colleagues have developed a flexible platform for contrast agents that can target specific cell types, such as macrophages, which are involved in the inflammatory response and plaque activation. ’Such agents could interrogate the blood vessel wall and even get inside plaques,’ he says, ’and become an important tool in MR or CT imaging of cardiovascular disease.’ Fayad describes the approach he has taken to novel contrast agents as ’plug and play’ molecular software, because it could in theory be adapted to many different targets for other medical problems.
Drug barriers
’Contrast agents greatly enhance existing imaging methods,’ Mulder says. ’The main hurdle is that contrast agents are actual drugs and therefore need FDA [US Food and Drug Administration] approval. This may limit their applicability in the clinic, especially because of the high costs that are involved.’
As imaging technology evolves, however, and with MRI machines now pulsing at three Tesla in many medical and research facilities, chemists must keep pace to allow this technology to be exploited to the full across medicine. Machines operating at three Tesla and beyond have rendered conventional agents inadequate. In order for biomedical researchers and clinicians to be able to exploit these new high-performance machines, chemists need to mix chemical expertise with biomolecular know-how to write new molecular software.
New agents are opening up areas of medical research previously inaccessible to imaging down to the cellular and even molecular level, allowing biochemical processes to be imaged in unprecedented detail. Other researchers such as Thomas Meade at Northwestern University, US, are even working on inorganic coordination complexes that will allow them to image the process of gene expression. Ultimately, imaging at this level will be combined with therapy, allowing clinicians to track changes at the molecular level as a drug takes effect.
The simple chelation complexes of paramagnetic ions will be supplanted by multimodal ligands that can carry ions to specific sites, operate on the microscale, and the cellular and molecular level with MRI, CT, and other imaging techniques. If ever the time was ripe for an upgrade it is now.
David Bradley is a science writer based in Cambridge, UK






No comments yet