Synthetic chemists are finally mastering the assembly of interlocked molecules held together by the mechanical bond, find James Mitchell Crow
The newest bond in chemistry might not be a chemical bond at all.
‘The mechanical bond isn’t something that you can really point to in space and say “This is the bond,”’ says David Leigh from the University of Manchester in the UK.
A mechanical bond is formed when one molecule is threaded through another, then cyclised or otherwise modified to trap the two components in a physically interlocked state – like two rings in a chain link fence. Compared to the other bonds in the chemist’s lexicon such as the covalent bond, or even non-covalent linkages like the hydrogen bond, mechanical bonds are quite unusual.
‘It’s a bond in that it holds two components together that would otherwise have independent degrees of freedom and fly apart,’ says Leigh, whose increasingly intricate interlocked molecules often incorporate multiple mechanical bonds in a single structure. ‘But it differs from other kinds of bonds because you don’t have intrinsic fixed limits to bond angles and bond lengths.’
There is a lot that’s different and special about the mechanical bond
Mechanical bonds are also unlike other chemical bonds in that they don’t involve charge or the sharing of electrons, adds Steven Goldup, who makes mechanically bonded molecules with chemical function at the University of Birmingham, UK. ‘The mechanical bond is literally just the inability of atoms and bonds to pass through one another,’ he says.
‘If you wanted to be really pedantic, and say that chemical bonds are about the sharing of charge between specify atoms, you probably would say it isn’t a bond,’ Goldup adds. ‘But the mechanical bond is a permanent interaction between two chemical entities that results in them not being able to separate – which feels like a bond,’ Goldup says. ‘It fulfills the macroscopic definitions of a bond.’
The mechanical bond’s unconventional nature – including the large amplitude motions it permits between bonded parts – is also its key appeal. Making mechanical bonds gives access to structures with properties that cannot easily be accessed any other way.
‘I think there is a lot that’s different and special about the mechanical bond,’ says Fraser Stoddart from the University of Hong Kong, who shared the 2016 Nobel prize for his work on molecular machines enabled by mechanical bonding.
Making a mechanical bond
Mechanically bonded molecules typically fall into two broad categories. If a linear molecule is threaded through a macrocycle and then cyclised to form a pair of interlocked rings, the resulting structure is called a catenane. If the threaded molecule is fitted with bulky stoppers at each end which prevent it from unthreading, the result is a rotaxane.
The elaborate rotaxanes and catenanes made today can make it easy to forget that, until surprisingly recently, even the simplest interlocked structures seemed out of reach. ‘In the 1980s and 1990s, threading molecules through each other just seemed virtually impossible,’ Leigh says. ‘The structures produced in that period by Stoddart and [Jean Pierre] Sauvage were absolutely amazing.’
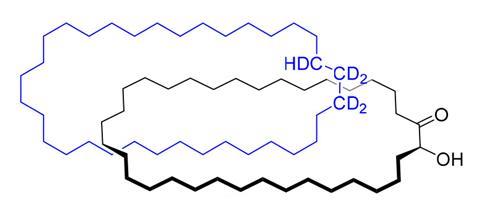
The earliest reported example of a synthetic mechanical bond – which Leigh recently revisited in his lab – illustrates the challenge. The concept of the mechanically interlocked molecule had been floating around for a while when, in 1960, Edel Wasserman from Bell Telephone Laboratories, US, played the odds.
Wasserman mixed a 34-carbon macrocycle with a long chain molecule of similar size, which he then cyclised. If the long chain just happened to be threaded through the macrocycle at that moment, a mechanical bond would result. ‘Wasserman’s idea was that maybe a molecule in a million will close with the thread through the ring,’ Stoddart says.
In a 1960 communication, Wasserman claimed he had detected traces of a mechanically interlocked pair of macrocycles made by this ‘statistical method’. But few were fully convinced by the experimental evidence Wasserman put forward.
In 2023, Leigh showed using modern spectroscopic methods that the catenane Wasserman claimed can indeed be formed by this reaction. ‘It vindicates that Wasserman’s claims are justified,’ Stoddart says.
The milligram or so of material Wasserman made from 10 grams of starting material wasn’t going to supply useful quantities of catenane, however. The next claim to catenane synthesis was even more remarkable, if no more practical. In 1964, Gottfried Schill from the University of Freiburg, Germany, published an approach called covalent templating. Using classical covalent bond chemistry he painstakingly constructed an interwoven polycyclic system, designed so that cleaving select covalent bonds in the last step of the synthesis would leave two rings held together only by a mechanical bond.
‘Once he had the bits and pieces linked by covalent bonds like acetyl bonds, he could hydrolyse them and he would have two interlocked rings, or a ring on a dumbbell,’ Stoddart says. Schill even went on to make molecular knots. ‘It was remarkable chemistry – I think he was worthy of a Nobel prize – but it was 20-odd step synthesis,’ Stoddart says. ‘So it was never really going to carry the day in terms of use.’
The key step forward in philosophy and methodology came in 1983. Like Wasserman, Jean-Pierre Sauvage of the University of Strasbourg in France started with a mixture of a macrocyle and a linear molecule. ‘Sauvage’s genius was to realize that you could use template effects to form threaded structures,’ says Leigh. Rather than rely upon chance association, Sauvage used metal ion templating to pre-associate the two starting materials, so that they were already in position when he cyclised the linear molecule to close the mechanical bond.
Following Sauvage’s advance, practical methods for making interlocked molecules, typically employing a templating or other associative interaction to hold the components in place, gradually began to appear.
Pi in the sky
The templating chemistry Sauvage adopted had its origins in macrocycle chemistry. ‘In the 1960s, even macrocycles were extremely difficult to make,’ says Leigh. ‘Being able to template things revolutionised that.’ This work was recognised by the 1987 chemistry Nobel, awarded to Donald Cram, Jean-Marie Lehn and Charles Pedersen.
It was an early Pedersen publication that set Stoddart on his own path toward a mechanical bonding breakthrough. In April 1967, just after starting his postdoc, Stoddart came upon Pedersen’s work in a brief communication. Petersen had reported the first crown ether, dibenzo-18-crown-6. ‘I decided, being the sort of person I was, that if Peterson could make 18-membered rings, then maybe I could make even bigger and better ones,’ Stoddart says.
Stoddart made several macrocycles, up to 35 membered rings, from truncated cone-shaped carbohydrates called cyclodextrins. ‘Then there was the disappointment, because they didn’t do anything when we tested them,’ Stoddart says.
As Pedersen was already showing, however, there was plenty you could do with crown ethers. These cyclic structures could host all manner of guest ions and molecules, as Stoddart also began to explore.
It wasn’t just the ether functionality of these macrocycles that could form non-covalent interactions with a guest molecule. One structure Stoddart made was a complex between dibenzo-30-crown-10 and a bipyridine platinum complex. The crystal structure revealed pi–pi stacking between an electron-rich benzene group on the crown ether and the electron-poor bipyridyl ligand of the platinum complex.
When the team subsequently assembled an all-organic host–guest complex between a crown ether and the linear organic molecule paraquat, the pieces clicked into place. ‘When we saw the relationship between the ring and the paraquat, it didn’t take much wit to see we were on the doorstep to the mechanical bond,’ Stoddart says. In 1989, the team exploited the pi–pi interaction between an electron-rich crown ether and the electron-deficient paraquat to assemble a catenane consisting of the crown ether mechanically interlocked with a macrocycle assembled from two paraquat p-phenylene units. ‘The yield of that first reaction was 70%. And you can now make it literally in 97% yield,’ Stoddart says.
A key feature of Stoddart’s structures, compared to Sauvage’s, was the strong pi–pi interaction between the component parts. ‘When Sauvage washed the copper out, that stopped the crosstalk between the rings, whereas our rings had a lot of crosstalk – and that meant that we could start thinking about making switches and ultimately machines,’ Stoddart says. In 1991, the team made a rotaxane version, which they called a molecular shuttle. ‘That first shuttle was a degenerate system that just went back and forth,’ Stoddart says. ‘But in 1994 we de-symmetrised it, to make the first rotaxane-based molecular switch.’ Myriad molecular machines followed.
Switches and machines were not the only way the motion afforded by interlocked molecules could be harnessed. In the early 2000s, Kohzo Ito at the University of Tokyo, Japan, invented a mechanically interlocked polymer which he called a slide ring gel. The material consisted of long polymer chains threaded onto cyclodextrins, forming mechanical crosslinks between neighbouring polymer chains rather than the usual covalent crosslinks. ‘When you stretch a normal polymer network, stress builds up in the crosslinks, and that’s where the polymer tends to break,’ Goldup says. ‘The slide ring gels allow the strain to equalize across the network, and so the network effectively gets stronger.’
Slide ring coatings featuring mechanical bonds have been explored as tough smart phone screens, and used in commercial products from golf ball coatings to sound absorption materials. They have even been investigated as stretchy binders for lithium-ion battery anodes.
Active template
Early in his independent academic career at the start of the 1990s, Leigh was looking to synthesise macrocycles that would absorb carbon dioxide from the atmosphere, when he accidentally made a catenane instead. ‘At that time, making catenanes and rotaxanes was extremely rare,’ he says. Rather than Stoddart’s aromatic stacking interactions or Sauvage’s metal ion templates, Leigh’s structures assembled due to hydrogen bonding. ‘So we thought, let’s see what we can do with those kinds of molecules.’
From the mechanical bond assembly point of view, arguably one of Leigh’s key contributions is his 2006 active template approach. ‘The active template turned mechanical bond formation from a supramolecular chemistry problem to a synthesis problem,’ Goldup says. The first active template systems took the idea of metal ion templates, and turned it into a catalytic process. The metal ion not only templated the association of the two components to be mechanically bonded, but catalysed the ring-closing step to form the catenane.
The latest iteration of this chemistry is the metal-free active template. Previously, most mechanically interlocked structures threaded themselves because they were designed to be the most thermodynamically stable structure, Leigh says. ‘Those are relatively easy to make,’ he says. Much more interesting would be to form threaded structures that are not the most stable structure, Leigh adds. ‘So how do you do that? Non-metal active template synthesis allows you to design molecules that will thread through each other, and the threading action causes them to react,’ he says. By stabilising the transition state, the threading action accelerates the cyclisation or stoppering group reaction. ‘They just intrinsically form these higher energy mechanically interlocked structures on their own.’
This chemistry is a world away from the original methods of Sauvage or Stoddart, which required many steps, were difficult to make, and required very specialist functional groups to be incorporated into the structures, Leigh adds. ‘Now, with things like active template synthesis where the template interactions don’t live on in the final product, you can make rotaxanes and catenanes out of almost anything,’ he says. ‘Making catenanes and rotaxanes is now completely routine.’
The intricately interwoven, multiply mechanically bonded molecules now being made illustrate how far the field has come. ‘In the last 10 years, the level of complexity of mechanically interlocked molecules people are making, and the yields they are achieving, have gone up massively,’ Goldup says.
The research emphasis now is on application. A growing number of synthetic organic chemists, polymer chemists and beyond are beginning to introduce mechanical bonds into their molecules.
Gold standard
In the early days of mechanical bond exploration, the emphasis was on molecular machines. ‘The mechanical bond is very mobile, and that caught people’s imagination,’ says Goldup, who spent several years as a postdoc in the Leigh lab making molecular machines. When Goldup started his own lab, he took a different approach. ‘I was interested in how we can use the mechanical bond to solve chemical problems,’ he says.
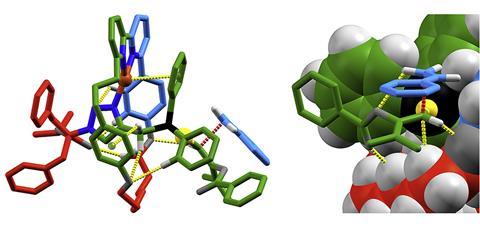
There’s more to the mechanical bond than the motion it permits between bonded parts. ‘A mechanical bond can be a very, very effective way of building up steric bulk,’ Goldup says. In a single step, making one mechanical bond results in a dramatic change in molecular shape that would take numerous covalent bond forming steps to reach. The resulting interlocked structure can be chiral even when assembled from two achiral starting structures. ‘You can use that for sensing and catalysis,’ Goldup says. ‘We’re trying to solve the sort of chemical problems that everyone does in synthetic chemistry, just from a slightly different perspective.’
One example is the enantioselective gold catalyst the team has developed. ‘Gold catalysis is generally hard to render enantioselective because you have a linear coordination geometry at the gold,’ he says. That means the substrate binds on the opposite side of the metal to the chiral ligand. But with an interlocked molecule, the gold can be embedded within the flexible cavity created by the mechanical bond. ‘We showed we got enantioselective catalysis, which was very exciting,’ Goldup says. Not because the catalyst was a world-beater, but because of the possibilities it suggests. ‘These things are now relatively easy to build, and in theory we could use it to solve catalysis problems that can’t easily be solved any other way.’
Going retro
How do you design and make a mechanically bonded molecule? ‘It’s essentially the same as for a complex natural product,’ Leigh says. The process starts with retrosynthesis – with the one key difference that the molecule is being designed for function, not structure. ‘If we do the retrosynthetic analysis and we realize that it’s much easier or cheaper to make the molecule if we include a methyl group, say, then we’ll put that methyl group in,’ he says.
With natural product synthesis you don’t have this structural flexibility. But once the molecule is made, the task is complete. With a mechanically bonded molecule, the finished product must do what it was designed for. ‘A molecular walker that doesn’t walk or a catenane that isn’t threaded, those things don’t tend to publish well,’ Leigh says.
Building a mechanically interlocked structure is now just another form of organic chemistry, Leigh adds. ‘Once you design your molecule, you go away and build it using the same tools, skills and reactions that you would use doing natural product synthesis or a drug synthesis,’ he says.
The active template approach has turned mechanical bonding into a form of organic chemistry, Goldup agrees. ‘You’re not thinking about binding constants, you’re not doing titrations, you just mix three components and you get the interlocked structure you intended to get,’ Goldup says. ‘The chemistry is now completely accessible.’
But few organic chemists so far have really embraced the mechanical bond. ‘If I was to go into an organic synthesis lab and say “Do you want to make a rotaxane?” I think most people would pull a face,’ Goldup says. That’s partly because the properties that mechanical bonds impart, and so the reasons for making one, are still being established, Goldup adds. ‘That’s now our job, I think, to show people why they should make them.’
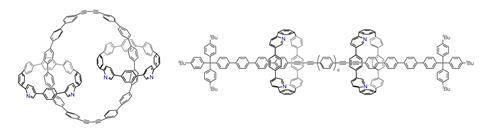
One synthesis group starting to explore mechanical bonding is Ramesh Jasti and his team at the University of Oregon, US. Since his postdoc days in Carolyn Bertozzi’s team at the Molecular Foundry in Lawrence Berkeley National Lab, US, Jasti has focused on carbon nanomaterial synthesis. ‘The one that really struck me was carbon nanotubes, which are very difficult to synthesise with control over the structure,’ Jasti says. He set out to assemble short sections of nanotube bond by bond, developing ways to make a carbon ‘nanohoop’, cyclioparaphenylene (CPP), with complete atomic precision.
The idea of linking pairs of these macrocycles with a mechanical bond had floated around the group for a while before the team had a go at making one. ‘The mechanical bond gives you the opportunity to make things that move based on stimuli,’ Jasti says. ‘If you bring that into the world of carbon nanostructures, which typically have more interesting electronic and optical properties but are more static structures, how might that manipulate the properties?’
Jasti used the active template approach to produce mechanically interlocked CPP molecules. ‘I think it was probably one of the most difficult things we’ve done,’ he says. ‘It took two exceptional graduate students pretty much their whole careers – they just devoured the literature to come up with a strategy and develop it to where it is now.’
The challenge was not the mechanical bond forming reaction per se. ‘If you make some of the structures that have been well explored, I think it can be very straightforward,’ Jasti says. ‘But the combination of our molecules and the mechanical bond is tricky,’ he says.
The effort already looks like it might pay off, however. The team has just begun to explore the properties of their mechanically bonded nanohoops, but already there are hints of unusual behaviour. ‘For example, we know that there’s very efficient energy transfer from one interlocked ring to the next,’ Jasti says. The team shone light at a wavelength tuned to one ring, expecting to see some light emitted by that ring and some light emitted by the other after energy transfer. ‘We only see an emission from the second ring, which must mean that the energy transfer is really fast,’ he says. ‘You could imagine one day maybe programming a system to systematically move charge or something down a long chain of these things.’
The team needs more material to test out some of the other properties they are interested in, but has already developed improved methods to make mechanically bonded CPPs at larger scale. ‘Right now, I don’t even think many people have even theoretically calculated the properties for these types of materials,’ Jasti says. ‘Now that they see it, I think theoreticians will dream up a lot of possibilities–- and then you’’ll see a lot of papers come out.’
James Mitchell Crow is a science writer based in Melbourne, Australia
Bonds are the ties that bind chemistry

Those seemingly simple sticks belie our most complex concept
- 1
- 2
 Currently
reading
Currently
reading
The mechanical side of bonding
- 4
- 5
- 6



























No comments yet