Our understanding of what an element is has evolved over the years, but it’s still a tricky concept to nail down. Philip Ball investigates
As chemical concepts go, you don’t get much more fundamental than the element. It’s one of the first ideas that the chemistry student encounters, often in the iconic tabulation of these basic ingredients of nature that Dmitri Mendeleev first described 150 years ago and which is being celebrated this year. And yet no one can say quite what an element is. The question was debated with much vigour and occasional passion during a meeting of the International Society for the Philosophy of Chemistry in Bristol in July 2018 – but still without producing any consensus.
That’s no surprise. Some of chemistry’s finest minds, including Antoine Lavoisier, Mendeleev himself, and pioneer of nuclear chemistry Frederick Soddy, have grappled with it, yet still a concise and comprehensive definition remains elusive. And some of the participants of the meeting implied that this might be for the best.
For others, it’s an indication that chemistry has some serious philosophical thinking to do. ‘Chemistry does not understand itself as a discipline,’ says philosopher Farzad Mahootian of New York University in the US. It’s not just the definition of an element; concepts such as molecules, bonds, and even the character of the periodic table itself, remain fuzzy: deceptively familiar from regular use by practitioners, but lacking any meaning that everyone agrees on. ‘There’s a need for philosophical reflection on aspects of chemistry that we tend to teach in a rather mechanical way,’ says Eric Scerri of the University of California Los Angeles in the US, editor of the philosophy of science journal Foundations of Chemistry.
It seems reasonable to expect chemistry to provide an unambiguous definition
The meaning of an ‘element’ is a favourite topic for argument among off-duty chemists. We agree (right?) that hydrogen is an element – but what do we mean by that? Is molecular hydrogen gas an element? Or the isolated hydrogen atom? Or are we referring not to some actual substance but to a ‘transcendental’ notion of hydrogen of which actual atoms and molecules are just material representatives?

Some might say: who cares? We know what we mean in practice. If I say ‘Sulfur is an element that forms a yellow solid with a pungent smell,’ I don’t expect objections. Likewise if I say ‘Sulfur is the second element in group 16 of the periodic table.’ But these are two rather different things.
According to theoretical chemist Eugen Schwarz of the University of Siegen in Germany, the typical attitude is to say ‘I know how I speak about elements isn’t really correct, but everyone does it, and students will figure it out in the end.’ But ‘my personal feeling as a chemist is that one should not adopt this habit’, he adds.
Elena Ghibaudi of the University of Turin in Italy worries that this failure to define an element precisely raises problems of understanding, communication and trust in teaching. ‘When two experts in chemistry are discussing elements, they are able to distinguish the meaning from the context, but this is not so in the classroom,’ she says.
There could be problems for the public understanding of chemistry too. Schwarz points to how, because some elements become associated with toxic substances – chlorine gas, say, or sulfur in the sulfur dioxide released from burning coal and oil – the element itself may become regarded as inherently toxic, and vulnerable to chemically illiterate bans. ‘I don’t know how to make it clear to the public that only some compounds of a given element are toxic, and even that only above some particular concentration, while too little of that same element may even cause health problems,’ he says.
‘The notion of an element is central to chemistry and serves a number of purposes,’ Ghibaudi says. ‘For example, it identifies what stays unchanged in a system undergoing a chemical transformation, and it distinguishes between chemical and nuclear changes. So it seems reasonable to expect chemistry to provide an unambiguous definition.’ Can it, though?
Earth, wind and fire?
Like the idea of atoms, elements suffer rather than benefit from an illusion of continuity in a long tradition of thought. The popular story has it that the ancient Greeks thought there were just four elements – earth, air, fire and water – but that from around the eighteenth century we began to appreciate that there are rather more than four, and that none corresponds to these ancient elements. The truth is more complex. For one thing, the four elements attributed to Empedocles and enshrined in Aristotle’s philosophy were by no means the only scheme for the basic building blocks of matter in Greek thought. And before the golden age of chemistry during the late Enlightenment, ‘element’ systems were rather hazy. The 16th century Swiss physician Paracelsus proposed three fundamental ‘principles’ sulfur, salt and mercury, while several other schemes (including such fictitious elements as phlogiston) enjoyed temporary support.
Should each isotope occupy its own place in the periodic table?

Besides, these weren’t necessarily competing alternatives. The idea of an element, like that of an atom, had a rather varied connotation, and didn’t necessarily mean a primal type of matter. The three principles of Paracelsus, for example, were seen more as properties than ingredients: sulfur representing combustibility, salt solidity and mercury fluidity.
Robert Boyle is rightly celebrated for bringing some clarity to the concept when, in his 1665 book The Sceptical Chymist, he proposed that an element was a substance that could not be reduced (‘analysed’) to something simpler. But Boyle’s definition only tells you when you have an element, and not what an element is and what distinguishes one from another. And it is highly provisional, hostage to your analytical capabilities. How could you be sure that you had an element and not just a compound that no one had yet found a way of splitting into its ingredients? Indeed you couldn’t, which is why oxides that are hard to split, such as alumina and silica, appear as elements in 18th-century lists like that in Antoine Lavoisier’s influential 1789 Traité Élémentaire de Chimie. Lavoisier followed Boyle in asserting that an element represents the final stage of analysis.
John Dalton brought something more fundamental to Lavoisier’s definition when he asserted in 1808 that the specific properties of elements derive from those of their constituent atoms, visualised as tiny, hard, spherical particles. By Mendeleev’s time in the middle of that century it was recognized that different elements have different atomic weights, and in drawing up his periodic table Mendeleev used an ordering of elements based on their atomic weight. (He himself used the term ‘elemental weight’, since he didn’t believe in atoms.)
The discoveries of radiochemists such as Soddy, and physicists like Ernest Rutherford and Henry Moseley, brought an understanding by the 1920s that the more fundamental property of an element’s atoms is their atomic number Z – the proton count of their nuclei – which is the same for all atoms of a given element. Francis Aston discovered isotopes in 1922, which have the same Z but different atomic mass. But if Z differs for two atoms, they’re different elements.
What do we mean by ‘carbon’? Diamond, an atom with Z = 6 or C60

At first, though, isotopes threw a cat among the pigeons. ‘Their discovery was a challenge for the definition of an element,’ says Ghibaudi. ‘There was an animated debate on the concept of the chemical element among chemists and physicists. The question was whether or not each isotope should occupy its own place in the periodic table.’ In 1923 an international committee agreed to base the identification of the chemical element on the atomic number instead of the atomic weight.
That, you might think, could have been the end of the matter: elements are defined by Z. The trouble is, this isn’t quite how chemists use the word. In a seminal paper on the definition of elements in 1932, the German chemist Friedrich Paneth admitted two different definitions, which he called Einfacher Stoff – typically translated as ‘simple substance’ – and Grundstoff, or ‘primary/basic substance’. The first refers to Lavoisier’s notion of real, physical stuff that can’t be reduced by chemical methods into more basic ingredients, the second to an abstract notion: ‘oxygen’, say, as a type of atom with Z = 8.
Ghibaudi doubts that we’ve got beyond Paneth’s dualism even now. Iupac currently gives a double definition of ‘element’ in its ‘Gold Book’ of chemical terminology, which says that the word can refer either to a ‘species of atom’ (which Ghibaudi sees as akin to Paneth’s ‘basic substance’) or, rather tautologically, to a ‘pure elementary substance’.
This double meaning is uncomfortable. Look up ‘oxygen’ on an elements website, and you’re likely to be told it has Z = 8, and perhaps a particular electronic configuration and position in the periodic table – but also that it is a highly reactive substance with the formula O2 and a boiling point of –183°C. According to chemist Mark Leach, who runs the chemistry resource website meta-synthesis.com, this is a sloppy conflation of two quite different kinds of data: one referring to Paneth’s ‘basic substance’ (an abstract ideal), the other to his ‘simple substance’ (a real substance). Surely that can’t be good?
What’s more, says Leach, our whole notion of the periodic table mixes the two awkwardly. We might imagine that it is a tabulation of ‘basic substances’ – which is pretty much how Mendeleev saw it. But the whole notion of periodicity refers to actual chemical properties of real stuff: the valency in chemical compounds, properties such as ionisation energy, metallic character and so forth. ‘If the basic substance has only the property Z, there is just a simple list,’ says Leach. ‘Where then does the structure of the periodic table come from?’
Some popular renditions of the periodic table even show photos of the ‘simple’ material forms of the elements: diamond or graphite for carbon, and so on. So it’s a confusing mash-up – and maybe it has to be. ‘You need a judicious compromise of both the basic and simple properties to construct it,’ says Scerri.
This is not a trivial matter. Arguments still rage, for example, over whether the elements below yttrium in group 3 should be lanthanum and actinium, or lutetium and lawrencium. The dispute comes down to whether you think the table should reflect ‘fundamental’ characteristics such as electronic configuration, or observable ones such as chemical behaviour. Those arguments become even more ambiguous once relativistic effects (due to the very high speeds of inner-shell electrons) start to play havoc with chemical periodicity among the human-made superheavy elements.
This is getting heavy

That’s not the only complication the superheavies introduce. The confusion about whether an element is ‘stuff’ or ‘concept’ stems from the fact that in the past they have been both. But does a new element really have quite the same claim to reality when it exists only as a handful of atoms that are stable for less than a second, as is the case with some of the newest artificial elements, such as tennessine? If elements are partly defined by their chemical properties, where does that leave elements that don’t exist long enough to engage in any meaningful chemical interaction, and which in any case are made only as highly charged ions that never acquire a full complement of electrons? ‘In what sense are they elements if they only last for a millisecond or two?’ asks Scerri. ‘I don’t think we’re ever going to be able to put them in bottles.’
In what sense are they elements if they only last for a millisecond or two?
Radiochemistry has always sat a little uneasily within chemistry’s stable. One common way of thinking about chemical elements is as the ‘conserved quantities’ of chemistry. Just as mass and energy are never destroyed in physics (although of course they can be interconverted), so chemistry’s fundamental conservation rule is that elements persist: you never exit a reaction with less carbon than you started with. But in radiochemistry, where one element may decay into another, you do. Whether this makes radiochemistry part of chemistry at all has been disputed since its early days, when the physics and chemistry Nobel committees competed for who should award prizes to the likes of the Curies and Rutherford (both of them now ‘claimed’ for chemistry in the names of elements).
That turf war has never ended, as witnessed by the latest squabbles between the International Unions of Pure and Applied Physics and Chemistry (Iupap and Iupac) over who should get to pronounce on the confirmation of new elements. Physicists say only they have sufficient expertise to judge the claims made from atom-smashing experiments with particle accelerators. But chemists are not happy to let another group determine what goes into their most prized icon, the periodic table.
Whoever does the adjudication, these new elements aren’t stuff you can hold in your hand. They highlight the new relevance of timescales. Arguably any merger of nuclei that lasts longer than the typical timescale of nuclear scattering, around 10–10 s, could qualify as the formation of another element. But does a union measured in nanoseconds really justify that, or is it merely a kind of resonance? This is why, Schwarz says, ‘when we talk about elements we should also talk about timescales’. He wonders whether an ‘element’ should at least be an entity capable in principle of forming molecules. ‘Chemistry is a craft and science of real materials,’ he adds – but ‘for physicists, a nucleus is an element’. Iupac, meanwhile, recently announced new criteria for the discovery of superheavy elements that affirm the timescale of existence to qualify for element status to be just
10–14s.
The thing in itself
The problem of elements shows that, as Scerri says, chemistry needs philosophy. ‘The issue of the “chemical element”, like some other issues in chemistry such as the notions of substance and structure, raises philosophical questions, and so cannot be sorted out without relying on ideas from philosophy,’ says Ghibaudi. In some ways the question goes right back to Plato, whose notion of ‘ideal’ intangible forms underpinned his view of actual physical entities. Paneth’s abstract ‘basic substance’ is also sometimes discussed in terms of Immanuel Kant’s notion of the Ding an sich – the ‘thing in itself’, or the fundamental aspect of reality beyond reach of our (fallible) senses.
Does the ‘basic’ definition of an element contain all the ‘simple’ characteristics within it?
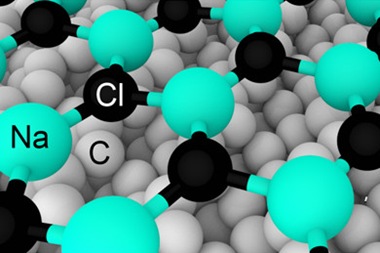
But if it’s a philosophical matter that can’t be resolved by empiricism, maybe we just have to make a choice between Paneth’s ‘basic substance’ and ‘simple substance’ as the definition of an element? Some researchers think so. Scerri, meanwhile, suggests that the nature of an element is not merely dual but threefold: what matters about the substance of an element is not just the properties of the raw stuff but the properties of its compounds. It is after all one of chemistry’s abiding wonders that, in sodium chloride, no trace remains of the reactive grey metal and the poisonous green gas.
Introducing a distinct nomenclature for the ‘simple’ and ‘basic’ definitions, so that dihydrogen molecules no longer be regarded as ‘the element hydrogen’, would require reform of deeply ingrained chemical language. But Sarah Hijmans of the Université Paris-Diderot in France questions whether we need to go to such lengths. Perhaps, she suggests, we might regard the word ‘element’ as one informed by both definitions. She says that in Lavoisier’s time there was no option but to go for the analytical definition, because we understood next to nothing about what distinguishes elements at the fundamental level. Gradually the balance has tipped more towards a ‘fundamental’ definition in terms of Z. But clearly the empirical, ‘chemical’ viewpoint still has worth, as the periodic table illustrates.
Perhaps the question is whether the two are actually in conflict at all. In one sense, there’s nothing terribly meaningful for chemists about Z at all, since the nucleus plays next to no direct role in chemical behaviour. The number of protons is just a proxy for what matters to chemistry: the number of electrons, as well as their configuration and energies.
But those, given a particular Z, are predetermined by the rules of quantum mechanics. They can be predicted. And from that information in turn we can in principle predict much chemical behaviour, such as what kinds of compounds the element will form. We can even predict the physical properties of some elements: allotropic forms, melting points and so on. So does the ‘basic’ definition of an element contain the all the ‘simple’ characteristics within it, to be revealed as our computational abilities get better?
Maybe, though, we have to accept that a certain vagueness will always surround the notion of an element. And perhaps that’s not so bad. Chemists, after all, are used to it – as Nobel laureate Roald Hoffmann has pointed out, they are forever using concepts that have no unique and precise definition, such as electronegativity and ionic radius, without that diminishing their value for the field. ‘Vagueness played a useful role in thought,’ says Mahootian. Maybe what matters is not vagueness itself, he argues, but to make sure it is not mere sloppiness.
So what, then, is carbon? The answer, says Schwarz, could depend on who we’re talking to. For different audiences and different purposes it could be soot; it could be element six, it could be a natural mixture of isotopes or a component of methane. Elementary, really.
Philip Ball is a science writer based in London, UK










No comments yet