Intermediate species ormed during a surface catalysed reaction have been imaged using atomic force microscopy (AFM), but those uncovered don’t match nicely with those predicted by theory. This has led the researchers behind the work to suggest that it would be worthwhile reconsidering our understanding of surface catalysis. Better knowledge of these kinds of reactions could aid the rational design of heterogeneous catalysts for industrially important reactions, the inner workings of which remain something of a mystery.
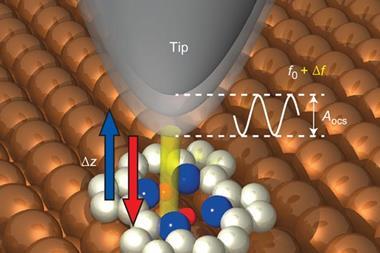
The way specific reactions occur on surfaces is crucial to industry. Most heterogeneous catalysts are solids, so a common industrial mechanism involves adsorption of the reactants onto the surface followed by reaction and desorption of the products. However, the details of the reaction kinetics, though often crucial to optimising the catalysts, are difficult to obtain. ‘Many of these big industrial processes are run on the level of understanding of a black box!’ says Felix Fischer of the University of California, Berkeley in the US. Non-contact atomic force microscopy, in which an atomically-fine metal tip oscillates above a surface at a frequency dependent on the force between the two, can build up images of individual atoms and chemical bonds. It takes minutes to build up an image, however, whereas individual chemical reactions can occur in femtoseconds.

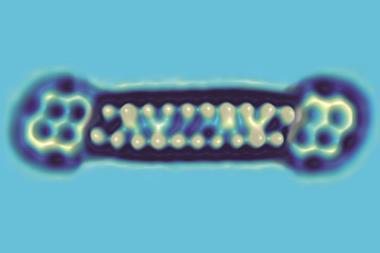
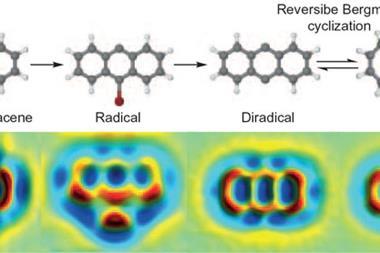
Fischer and colleagues looked at the bimolecular coupling of 1,2-bis(2-ethynyl phenyl)ethyne molecules and the subsequent isomerisation of the resulting dimers. They deposited the molecules onto a silver surface before cooling the reaction to cryogenic temperatures and analysing the quantities of the various molecules present using AFM. They then performed a series of further rapid cycles of heating and cooling. After each cycle, they conducted an AFM scan of the surface to determine the molecules present.
The researchers calculated theoretically that the reaction proceeds by an eight-step mechanism, and worked out the energies of the various intermediates involved. However, they observed only two of these. Neither of the standard approximations used to calculate reaction rates on surfaces predicted the stable intermediates and their quantities correctly – instead, the researchers had to consider the different rates at which various intermediates dissipate heat to the substrate and the change in entropy throughout the reaction. ‘We really have to rethink whether, with all these general ideas, we describe surface catalysis correctly in the literature,’ says Fischer.
‘This is a completely novel method of looking at this,’ says surface scientist Leo Gross at IBM Research in Zurich, who was not involved in the research. ‘And the main finding is that the potential energy landscape is not enough to understand which intermediates are stabilised. In addition, both selective dissipation and entropy have to be taken into account.’ Philip Moriarty of the University of Nottingham, UK, is also impressed: ‘Taking into account the entropic aspects of this, plus the energy dissipation, and coupling that back into the really high-resolution AFM is pretty impressive,’ he says.
References
A Riss et al, Nat. Chem., 2016, DOI: 10.1038/nchem.2506



















No comments yet