Battery buffer takes the strain
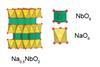
Unconventional layered oxide that counteracts volume expansion in electrodes could help batteries last longer

Unconventional layered oxide that counteracts volume expansion in electrodes could help batteries last longer