Close menu
- Home
- News
- Research
- Industry
- Opinion
- Features
- Culture
- Careers
- Podcasts
- Webinars
-
Collections
- Back to parent navigation item
- Collections
- Framing the future: policies to catalyse India's innovation ecosystem
- Energy storage and batteries
- 2025 in review
- Women's health
- Solutions for India's sustainability challenge
- The future of analytical chemistry
- Chemistry of the brain
- Water and the environment
- Chemical bonding
- Antimicrobial resistance
- AI and automation
- Sustainability
- Research culture
- Nobel prize
- Food science and cookery
- Plastics and polymers
- Periodic table
- Coronavirus
- Members
C50 breaks all the rules
By Victoria Richards2015-10-15T00:00:00
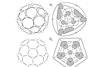
Are we any closer to a 3D analogue of benzene?
- This website collects cookies to deliver a better user experience. See how this site uses cookies.
- This website collects cookies to deliver a better user experience. Do not sell my personal data.
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies.
Site powered by Webvision Cloud