Chemically beheading per- and polyfluoroalkyl substances (PFAS) with inexpensive solvents and reagents destroys these persistent compounds. Although the method isn’t ready for field applications yet, experts say it is a promising start to address the problem of these bioaccumulative pollutants.
PFAS are ‘forever chemicals’ that do not degrade in the environment or human body. They are linked to a host of health issue like immune system dysfunction, low birth weight, high cholesterol, thyroid disease and certain cancers.
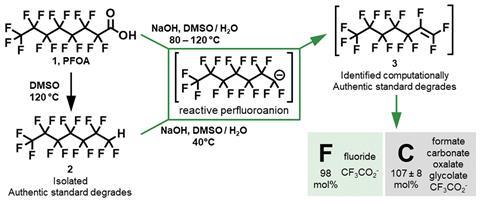
But researchers have discovered ‘previously unrecognised pathways that cause the entire molecule to fall apart in a cascade of complex reactions that ultimately produce the benign products’, explained study leader Will Dichtel from Northwestern University, US, at a virtual media briefing.
Heating PFAS with sodium hydroxide to 80–120°C in a water/dimethyl sulfoxide mixture first removes the molecules’ carboxylic acid head groups, leaving behind a reactive perfluoroalkyl ion tail. Within 24 hours, it further degrades to fluoride ions and small carbon-containing ions like formate and carbonate.
The researchers were able to degrade 10 perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl ether carboxylic acids (PFECAs) – including perfluorooctanoic acid (PFOA) and its hexafluoropropylene oxide dimer acid replacement GenX.
But the Northwestern team realised that many of their observations were inconsistent with what is known about PFAS degradation. In computational experiments, collaborators Ken Houk at the University of California, Los Angeles, US, and Yuli Li at Tianjin University in China found that PFAS did not fall apart one carbon atom at a time but rather two or three at once.
While other PFAS destroying technologies may need temperatures as high as 400°C, this one only works at low temperatures. ‘Most other technologies [in this arena] can be generally summarised as large chemical or energy inputs that degrade almost everything that is there,’ Dichtel explained.
However, the method does not destroy perfluorooctane sulfonic acid (PFOS) and other sulfonates, which are among the most bioaccumulative and toxic PFAS that have been studied. The US Environmental Protection Agency has identified roughly 12,000 PFAS on the planet, only a tiny fraction of which are characterised or well understood.
But Dichtel said other PFAS classes are likely to have their own weaknesses that can be exploited and then activated. Arjun Venkatesan, an environmental chemist and engineer at Stony Brook University, US, agrees. ‘Although the study is focused on only a subgroup of PFAS, perfluorocarboxylic acids, the success of the method allows the research community to explore the degradation of other PFAS, especially the more persistent perfluoroalkyl sulfonates,’ he tells Chemistry World.
Michael Wong, who chairs the chemical and biomolecular engineering department at Rice University, US, is most excited about the discovery of a new chemical pathway that breaks down PFOA without forming the usual shorter-chained derivatives. These are commonly seen in other approaches to destroy PFAS, like his own team’s photocatalysis work.
But Wong says it is unclear whether this method could be used directly in the environment, for example to treat river water. He suggests that it could be helpful for cleaning materials like activated carbon or ion-exchange membranes. These are used to decontaminate water but then become saturated with PFAS. ‘The spent material is generally disposed of in landfills or incineration,’ Wong notes. ‘This new chemistry allows for the PFAS contained in the solvent wash to be destroyed in a potentially cheaper way than other destructive methods being studied.’
Frank Leibfarth, a chemist at the University of North Carolina Chapel Hill, US, emphasises that this technology will not enable PFAS to be destroyed in water at environmentally relevant concentrations. ‘This is focused on decomposition of PFAS at high concentrations – orders of magnitude higher than those found in the environment – in organic solvent,’ he says. But finding ways to reduce the amount of base and use solvents that are more environmentally friendly and less costly would improve the method’s practicality, he says.
References
B Trang et al, Science, 2022, 377, 6608 (DOI: 10.1126/science.abm886)

















No comments yet