Researchers believe they have produced the long-sought after substance, but many are sceptical
For over a century, scientists have said it should be possible to turn hydrogen into a metal. Now, a pair of chemists in Germany claim to have finally performed the feat, although others remain sceptical.
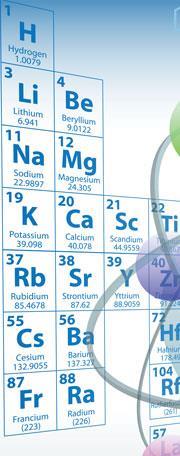
In the late 19th century, chemists pointed out that hydrogen, topping the column of alkali metals in the periodic table, ought to form a metal itself. Physicists Eugene Wigner and Hillard Bell Huntington predicted in 1935 that hydrogen should become a metallic solid at very high pressures - around 25GPa - but experiments later performed at these pressures showed no trace of a metal transition. More recent experiments, reaching pressures above 100GPa and temperatures approaching absolute zero, have offered hints of a metal transition, but generally results have been inconclusive.
The challenge of metallic hydrogen is alluring, partly because it has the potential for some exciting applications. Some believe studies of the material could lead to a room temperature superconductor, which would enable lossless power transmission. And if it is shown to be metastable, metallic hydrogen - being far denser than normal hydrogen - might make an efficient rocket fuel.
Mikhail Eremets and Ivan Troyan of the Max-Planck Institute for Chemistry in Mainz now believe they are the first to offer conclusive evidence for metallic hydrogen. They condensed hydrogen in the hole of an alumina-epoxy gasket, which sat inside a diamond anvil cell. They could measure light transmission with a laser directed through the diamond and into the gasket’s hole, and resistance via electrodes on the diamond surfaces.
At room temperature and 220GPa, the researchers found that the hydrogen appeared to become opaque and electrically conductive. Then, lowering the temperature to 30K at pressures above 260GPa, they found that the resistance increased by 20 per cent before levelling off. ’We found that [hydrogen] conducts to the lowest measured temperatures of 30K, and the resistance is nearly temperature independent, as [it] should be for metals,’ say Eremets and Troyan.
For Eremets and Troyan this effect - a subtle increase then plateau of resistance towards low temperatures - is the hallmark of metallicity. However, Arthur Ruoff, a materials scientist at Cornell University, New York, and one of the leaders in the search for metallic hydrogen, believes normal metal behaviour would have been more drastic: a resistance increase not of 20 per cent, but of 4000 per cent. He also suspects the hydrogen could be reacting with the gasket’s epoxy material, which would mean the results were in fact stemming from a hydrogen compound. ’There are so many questions,’ he says.
William Nellis, another leader in the search for metallic hydrogen at Harvard University, US, agrees that there are unanswered questions, including whether the gasket itself becomes conducting and reflective at high pressures. ’In view of the history of this field, Eremets and Troyan have a particular responsibility to demonstrate clearly to their colleagues that they have in fact metalised hydrogen,’ he says. ’In my opinion this demonstration has yet to be provided.’
Jon Cartwright
References
M I Nat. Mater., 2011, DOI: 10.1038/nmat3175








No comments yet