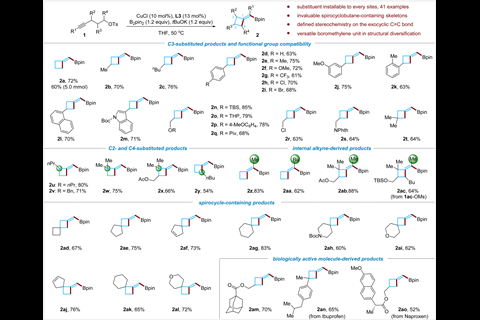
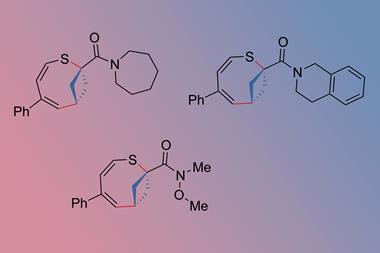
Researchers in China have developed a method for synthesising a variety of methylenecyclobutane derivatives. It overcomes previous synthetic challenges surrounding regioselectivity, stereoselectivity and functional group tolerance, so could make these underexplored compounds more accessible to medicinal chemists.
‘[Methylenecyclobutanes] are very small and strained compounds and are almost flat… so they are interesting structures,’ explains Bo Su, who led the work at Nankai University. They ‘have more reactive sites than cyclobutane because of the exo carbon–carbon double bond,’ which would allow them to be decorated with a range of functional groups.
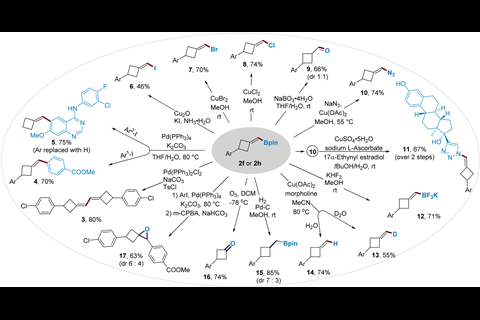
The method developed by Su’s team transforms readily available linear alkynes into (boromethylene)cyclobutanes via a copper-catalysed borylative cyclisation reaction. It requires relatively benign reaction conditions – 50°C for a maximum of 20 hours – and achieves excellent yields. Mechanistic studies found that an N-heterocyclic carbene ligand on the copper catalyst helps achieve high β-regioselectivity in a borylcupration step and promotes strained ring-closure in a vinyl copper intermediate. Su’s team also demonstrated how the boromethylene groups could be converted into a range of other functional groups.
Chuang-Chuang Li, whose group works on synthesising strained organic compounds at the Southern University of Science and Technology in China, describes the method as practical and selective. However, Li also warns that ‘functional compatibility may be an issue when the adjacent atoms are not hydrogen. Elimination will be a significant side reaction… the application of this methodology may be limited by the side reaction.’
Su says ‘there is one limitation; we cannot make them asymmetrically’. ‘If we cannot make this process enantioselective then it is a big limitation’.
Even so, Li says the method means ‘we will see more molecules bearing cyclobutanes and other strained moieties playing a more important role in medicinal chemistry.’ He hopes it will inspire new methods for synthesising novel and strained natural products.
Su is excited to build on the success of this work. On top of making the process enantioselective, which is the group’s primary focus at the moment, he wants to adapt it for broader cyclobutane derivatives. ‘There are much fewer natural products containing methylenecyclobutane… there are a huge number of cyclobutane-containing natural products,’ he adds.









No comments yet