Researchers in China have synthesised a series of molecules that resemble supersized versions of cyclobutane, cyclopentane and cyclohexane.
Cycloalkanes adopt puckered conformations to help relieve the strain caused when sp³-hybridised carbon atoms are forced into a ring and deviate from the ideal tetrahedral geometry. While these geometric quirks make cycloalkanes intriguing, studying the dynamics of their conformations has long posed experimental challenges.
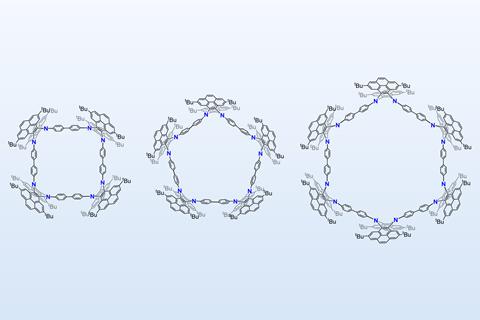
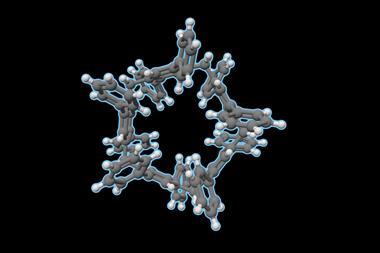
The polygonal macrocycles created by Zhaohui Wang and co-workers at the East China University of Science and Technology may offer a new way to unpick the conformational dynamics of cyclobutene, cyclopentane and cyclohexane. Their molecules feature four, five or six dipyrene-fused dihydropyrazine corners, which introduce flexibility and allow the rings to adopt low-energy conformations similar to those of classic cycloalkanes.
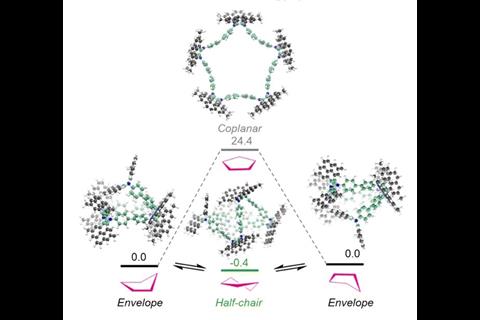
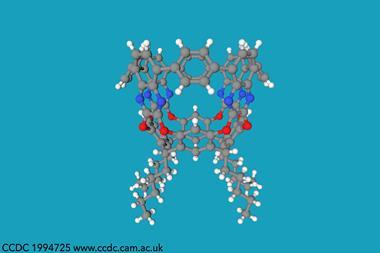
Yamamoto coupling reactions afforded the cyclic compounds in low yields. Remarkably, the scientists obtained single crystals of all of them. X-ray diffraction analysis confirmed that the macrocycles mimic the conformations of cycloalkanes: the four-membered ring adopts a puckered shape like cyclobutane, the five-membered ring takes on a half-chair envelope similar to cyclopentane, and the six-membered ring adopts the familiar chair conformation of cyclohexane.
The researchers also investigated how the four- and five-membered macrocycles change shape in solution, using temperature-dependent ¹H NMR spectroscopy. Supported by theoretical calculations, these experiments established the mechanisms and dynamics behind the conformational changes.
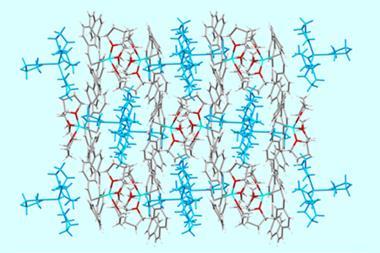
The second part of the work is focused on the supramolecular interaction of the new supersized molecules with fullerenes. Once more, the scientists grew single crystals to study these complexes. Analysis of the structures proved the interaction happens via π-π stacking with the pyrene units decorating the corners of the macrocycles. These supramolecular assemblies present a photoinduced charge transfer from the pyrene to the fullerene, suggesting potential applications in materials science.








No comments yet