Ethylene – a key feedstock in plastic manufacturing – is among the five top-selling compounds in the chemical industry. Now, two teams have independently discovered new electrocatalytic processes to prepare pure ethylene selectively and efficiently. Importantly, these reactions work under ambient conditions using abundant copper catalysts, providing a greener alternative to traditional solutions.
Most ethylene comes from steam cracking – a process that breaks down hydrocarbons into smaller molecules – and subsequent separation by distillation. However, ethylene streams contain small amounts of acetylene, an undesirable byproduct. ‘Acetylene impurities rapidly poison Ziegler–Natta catalysts during the polymerisation of olefins,’ explains Jian Zhang from North-western Polytechnical University in Xian, China, lead author of one of the papers.1 ‘This sharply decreases the quality of the target polymers … impurities in polymer-grade ethylene must be reduced to less than 5ppm.’ Their novel electrocatalytic approach transforms acetylene impurities into ethylene, using water as the hydrogen source.
‘This process is much more cost effective and environmentally friendly,’ Zhang adds. ‘We have achieved continuous production of polymer-grade ethylene streams, at large space velocity [and] under ambient temperature and pressure.’
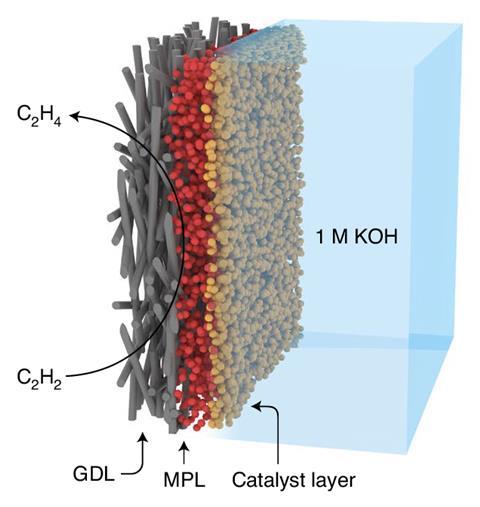
Traditionally, acetylene is removed using a thermal catalytic hydrogenation reaction that requires high temperatures and expensive palladium catalysts. ‘Besides, thermal catalysis requires excessive hydrogen, which is generally produced from reforming,’ says Run Shi, from the Technical Institute of Physics and Chemistry in Beijing, China, co-first author of the second paper.2 This reliance on fossil fuel hydrogen makes the purification process particularly polluting, as it generates vast amounts of carbon dioxide. ‘[Our] electrochemical process shows special advantages in energy and atom economy,’ Shi adds.
Furthermore, these new electrocatalytic solutions also enhance selectivity and efficiency. ‘Since thermal methods often rely on excess amounts of hydrogen, ethylene is often further hydrogenated into ethane,’ explains Gastón Larrazábal, an expert in catalysis and electrochemistry at the Technical University of Denmark. ‘This approach avoids this classic limitation of thermocatalysis, successfully removing the acetylene contamination.’
Scaling-up
For Larrazábal, the main breakthrough is the implementation of innovative gas-diffusion electrodes. ‘They enable acetylene better access to the catalyst’s reactive sites – making efficient electrochemistry with reagents in the gas phase,’ he explains. Both teams agree: the new gas diffusion approach offers a robust alternative for the purification of ethylene. ‘We’re opening a new research field in ethylene-related electrochemistry,’ says Shi.
Researchers are targeting copper as it is an abundant, sustainable alternative to palladium. ‘As far as we know, copper is the best catalyst,’ says Zhang. His team carried out both experimental and theoretical screenings to confirm this hypothesis, and compare copper to palladium, silver, gold and nickel. ‘Earth-abundant copper demonstrates the best activity and selectivity for electrocatalytic acetylene semi-hydrogenation,’ adds Zhang. Nevertheless, further developments in materials will certainly come soon. ‘The composition of the catalyst, the stability of gas-diffusion electrodes and the construction of alkali solid state electrolytes will be the next breakthroughs,’ explains Shi.
Despite their novelty, these devices should be easily scalable, as ‘the equipment is similar to fuel cells’, says Shi. ‘This technology enables high mass transfer between the gas phase and the catalyst, thus higher reaction rates – there is great potential for industrial implementation,’ says Larrazábal.
Zhang and collaborators are already scaling up their process, moving to electrodes that are 20 times bigger. ‘This will boost the max space velocity,’ he says. ‘Then we will connect multiple devices in parallel to promote industrialisation,’ explains Zhang. ‘We are making great efforts to optimise their stability … to more than 1000 hours.’
‘This is a novel, cleaner way to purify ethylene for polymer production,’ says Larrazábal, who also remarks the source of carbon is still non-renewable. ‘All the hydrogen atoms come from water, killing two birds with one stone: avoiding an extra step while using a green hydrogen source,’ he adds. ‘And it’s really cool to see researchers exploring new frontiers of electrocatalysis, even beyond CO2 reduction.’
References
1 J Bu et al, Nat. Catal., 2021, DOI: 10.1038/s41929-021-00641-x
2 R Shi et al, Nat. Catal., 2021, DOI: 10.1038/s41929-021-00640-y













1 Reader's comment