A new electrophotocatalytic method broadens the scope of the classic carbonyl olefination reaction. The approach works with a wider array of starting materials and produces less waste than traditional methods like the Wittig reaction.
Olefins are important building blocks for organic synthesis. But established methods of accessing these compounds from cheap carbonyl feedstocks often require strong bases, which limit the reactions’ functional group tolerance. Now, Tristan Lambert and graduate student Keri Steiniger from Cornell University, US, have instead employed electrophotocatalysis to access a greater range of olefins while simplifying the waste stream.
The process couples electrochemical and electrophotocatalytic oxidations to generate highly reactive intermediates in a controlled manner. The process enables readily available alkenes, themselves acting as olefination agents, to react with both aldehydes and ketones. The main by-products are nitrogen and hydrogen gases.
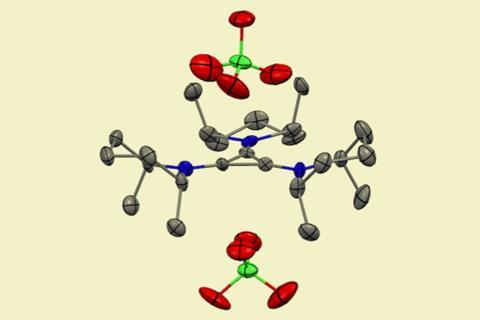
A powerful photooxidant catalyst allows the transformation to take place over a competing carbon–carbon bond-forming reaction, explains Lambert. ‘This project began by asking whether our TAC electrophotocatalyst could induce the oxidative denitrogenation of diazenes but with a product selectivity different than what was typical for such reactions,’ he says. ‘We speculated that controlling the rate of back electron transfer might enable conversion to olefins rather than cyclopropanes. That turned out to be true and the key, we believe, is due to the redox characteristics of the TAC.’
First, a diazo compound is electrochemically generated from an organic carbonyl and a hydrazine. A cyclic diazene then results from cycloaddition with the chosen alkene. The loss of nitrogen gas via electrophotocatalysis results in a distonic radical cation before reduction to the final olefin product. ‘It looks like a bit of a Rube Goldberg setup when drawn out on paper, but remarkably it all works together pretty well,’ adds Lambert.
Thierry Ollevier, an expert in homogeneous catalysis from Université Laval in Quebec, Canada, praises the approach. ‘With nitrogen and hydrogen as the main by-products, this new method has a major advantage over standard olefination methods [that usually lead] to problematic waste,’ he says. ‘Using in situ generated diazoalkanes paves the way to other applications involving subsequent reactions of unstabilised diazoalkanes. Scaling up can be envisioned.’
As an alternative to Wittig olefination, the new strategy offers broad applicability for synthesis of materials, pharmaceuticals and natural products. ‘This innovative method offers a real alternative to classical olefination, especially to make alkene targets that include base-sensitive functionality like alkyl halides or acetates,’ notes William Unsworth, an expert in synthetic organic chemistry from the University of York, UK. ‘The ever-growing prominence of electrochemical synthesis means that the method should be well used in organic synthesis in industry and academia.’
References
Reference: R A Steiniger and T H Lambert, Sci. Adv., 2023, DOI: 10.1126/sciadv.adg3026











No comments yet