Chemists have reported a rare instance of an energetically unfavourable reaction powered by a chemical fuel. The team took inspiration from synthetic biology, using a ratchet mechanism to drive the transformation.
For a chemical reaction to be spontaneous, it needs to release energy. This is why spontaneity, in this context, is typically reserved for exergonic reactions – those that release energy on their own. The idea behind a ratchet mechanism is to couple an energy-demanding process with an orthogonal energy input, which provides the energy necessary to satisfy the conservation of energy.
‘Random dynamic processes happen all the time in chemistry, from conformational changes to random thermal motion to reversible steps in chemical transformations,’ explains David Leigh at the University of Manchester. ‘Ratchet mechanisms give chemistry direction.’
Synthetic chemists have shown ingenuity, developing workarounds using catalysts and light to obtain the products of energetically unfavorable reaction pathways. However, these methods still fall short compared with natural processes. ‘It is no coincidence that … many important anabolic processes – the construction of complex molecules from simpler building blocks – harness the energy they need from chemical fuels,’ Leigh explains.
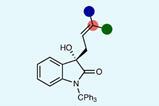
Leigh and colleagues demonstrated the selective transduction of energy from a chemical fuel to drive an energetically unfavourable Diels-Alder reaction – a reaction in which a conjugated diene reacts with a partner that contains a double or triple bond to form a six-membered ring. The fuel in this case is a carbodiimide to urea transformation that is catalysed by carboxylic acid groups present on the Diels-Alder starting materials.
‘The diene and dienophile [starting materials] … each have additional carboxylic acid “handles” attached,’ explains Leigh. ‘The fuel is used to tether the two building blocks together to form an intermediate anhydride.’ This brings the Diels-Alder starting materials into close proximity and drives the otherwise prohibited cycloaddition through an energetically downhill reaction via this tethered intermediate.
‘At the steady state of the fueled reaction, it is differences in the rates of reaction of different species with the fuel that cause the Diels-Alder reaction to proceed, not preorganisation of the building blocks,’ explains Leigh. ‘That is rather profound and fundamentally different to how chemists have carried out energetically uphill transformations in the past.’
A modest yield of 15% was reported after two fueling cycles with high levels of control. The team also say that the synthesis of the unfavourable Diels-Alder product is up to thirty times more energy efficient than a number of previously reported light-driven reactions.
‘Chemists have gained a new driving force to access energetically uphill transformations,’ comments Aleksandra Holownia, a process chemist working in the US who was not involved in the study. ‘By redefining the energy landscape, we can achieve energetically unfavourable chemical transformations, address issues of selectivity and design new synthetic methods that cannot be achieved under conventional controls.’
Holownia emphasises that while ‘conceptually this report gives synthetic chemists a new way to think about chemical transformations, [it] suffers from efficiency [problems as] the chemical fuel is unable to provide the Diels-Alder product in synthetically useful yields. An energy transducer that imparts a stronger influence to drive endergonic processes is key.’
Leigh says that increasing the number of cycles or the effectiveness of the Diels-Alder reaction in the tethered state could both increase the yield. However, in agreement with Holownia, increasing the yield of the reaction through the ratchet state would be more interesting.
‘It requires maximising the reaction rate differences between the reactants and products with the fuel, and the rate differences in hydrolysis between the tethered diene and dienophile and the Diels-Alder adduct anhydride,’ he says. ‘That is called the “kinetic asymmetry” of the reaction cycle. [It] is the key to both increasing the amount of energy to be transduced from the fuel-to-waste reaction and increasing the yield of the Diels-Alder [product].’
While more work lies ahead, Leigh says he hopes that these initial results will open up the synthetic chemistry toolbox, prompting more use of chemical fuels to supply the energy needed for endergonic synthesis, just like biology.
References
E Olivieri et al, Nat. Synth., 2024, DOI: 10.1038/s44160-024-00493-w












No comments yet