A fast flow chemistry method makes the production of sulfuryl fluoride quicker and safer. This gaseous reagent is useful in the synthesis of click-ready reagents, then easily functionalised into a myriad of chemical compounds, including peptides and proteins. Most reactions take place in less than two minutes, in a site-selective manner – a great opportunity for high-throughput experiments and drug discovery.
Sulfuryl fluoride is a surprisingly useful reagent in click-chemistry reactions, in particular the sulfur(VI) fluoride exchange, also known as SuFEx. ‘SuFEx chemistry is very similar but orthogonal to the more famous azide–alkyne click chemistry,’ explains Nina Hartrampf, an expert in flow synthesis for chemical biology applications in the University of Zurich, Switzerland. ‘The reaction proceeds under mild reaction conditions and often yields clean transformations,’ she adds. In SuFEx reactions, ‘two nucleophiles can react with sulfuryl fluoride and ultimately the SO2 moiety ultimately serves as a very small linker’ between the clicked fragments. ‘SuFEx click reactions are often used for the generation of libraries in medicinal chemistry [and] recently they have also found applications in the functionalisation of biomolecules such as peptides and proteins,’ says Hartrampf. Another attractive aspect of SuFEx reactions is versatility – they work with alcohols and amines natively, without the need for azides or alkynes, which is the case in copper-catalysed click reactions.
Researchers generate sulfuryl fluoride in situ by passing commercially available sulfuryl chloride through a reactor packed with solid potassium fluoride. The exchange of halogen atoms is driven by the higher stability of the sulfur–fluoride bond. ‘Our microreactor ensures the constant containment of the gas,’ explains lead author Timothy Noël, from the University of Amsterdam in the Netherlands. ‘This enhances safety … enabling the immediate consumption of the gas [and] significantly speeds up the gas–liquid mass transfer,’ he adds. The efficiency of the flow-system beats traditional methods, which rely on gaseous reagents that are unselective and difficult to handle safely.
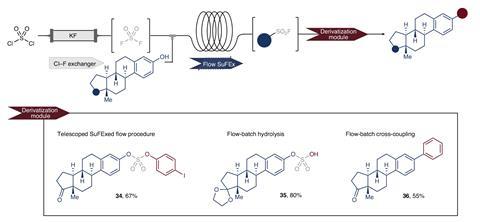
The preparation of sulfuryl fluoride from the combination of sulfuryl chloride and potassium fluoride was actually already known, explains Oliver Kappe, an expert in flow chemistry based at the University of Graz, Austria. However, ‘the main breakthrough is the on-demand synthesis of sulfuryl fluoride using flow chemistry’, he says. ‘The packed-bed reactor [makes] the process faster and more selective towards sulfuryl fluoride.’ This compound is ‘a mildly toxic gas, which most bench chemists would [avoid] … as it’s difficult to accurately dose and source in small quantities,’ adds Kappe. This in situ set-up ‘is a well-established principle in flow chemistry – the “chemical generator”, whereby a hazardous reagent is made on demand, on the fly, and subsequently used’.
Usually, chemists try to avoid gaseous reagents replacing them with crystalline alternatives that are easier to handle. ‘However, this increases the molecular mass … [and] leads to the production of numerous byproducts [and] raises the cost,’ says Noël. ‘Moreover, since these reagents are ultimately derived from sulfuryl fluoride, it merely defers the problem.’ The new flow method is aligned with the principles of atom-economy and green chemistry, boosting efficiency, and reducing waste.
‘This method [could] be particularly useful for medicinal chemists, who could apply SuFEx chemistry in their drug discovery programmes to functionalise molecules in a highly diverse way,’ says Kappe. The substances prepared in the paper, ‘could be further manipulated into molecules with interesting biological functions’, he adds. Additionally, the modularity of the method means ‘most synthetic chemists with minimal knowledge of flow chemistry and specialised equipment’ could successfully synthesise SuFEx reagents.
Hartrampf is ‘very impressed by the versatility of the method and … the extremely rapid and highly selective modification of peptides and proteins’. Indeed, the flow strategy developed by Noël’s team successfully and selectively functionalises peptides and proteins. ‘We gradually expanded the range of our approach starting from small molecules to drug-like structures, and finally to peptides and proteins,’ he explains. ‘Despite their sensitivity and numerous functional groups, these biomolecules [seemed] tolerant to our chemical process,’ adds Noël. ‘We find this aspect quite fascinating and plan to further explore it soon.’
References
M Bernús et al, Nat. Synth., 2023, DOI: 10.1038/s44160-023-00441-0
















No comments yet