Linde group launches ink made with long, individual tubes
Industrial gas specialist Linde Group has branched out into carbon nanotubes, launching SEERe- ink. The ink contains long, individual single-walled nanotubes suspended in dimethylsulfoxide. The company is targeting R&D markets for sensors and electronics applications, as well as touchscreen display manufacturers.
The process for making the ink was developed by researchers at the London Centre for Nanotechnology, led by Milo Shaffer, Neal Skipper and Chris Howard.1 They discovered that nanotubes can be reduced in solutions of liquid ammonia and alkali metals, to make salts of negatively charged ‘nanotubide’. The ammonia can then be removed and the nanotubide salts dissolved in a polar solvent to make the ink.
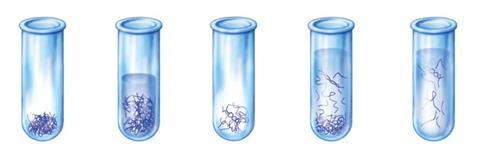
Shaffer explains that the negative charge on the tubes makes them repel each other and keeps them separated in solution. The process also avoids sonicating or oxidising, which is usually used to disperse nanotubes, but also ends up damaging them and making them shorter, which affects their properties. ‘But if we start with long tubes, we end up with long tubes in solution,’ Shaffer says. That’s crucial for applications in electronics, he adds, because junctions between tubes increase resistance, so longer tubes means fewer junctions and better conductivity.

But to move from the laboratory into a scalable, safe and reproducible industrial process required a partner, and that’s where Linde came in. ‘If you want to scale up something that involves large quantities of ammonia, then that’s a gas company sort of technology,’ Shaffer says.
Inking the deal
Sian Fogden, a former post-doc in Shaffer’s group and now the market and technology development manager at the newly-established Linde Electronics Nanomaterials division, explains that Linde’s setup is very similar to the laboratory rig. There are a few small tweaks, she says, such as the way the ammonia is removed after the nanotubide salt is formed. As demand grows and the process is scaled up further, other concerns will need to be incorporated, such as recycling materials where possible.
Linde is initially targeting the ink at research-based applications, Fogden says. Some of these are areas where nanotubes are already being used, such as sensors, displays, thin film conductors and composites. Others are looking to take advantage of some of the unique features of the nanotubide salts. For example, she adds, the charge on the tubes allows them to be functionalised very precisely, without disrupting the carbon framework. ‘We’re also pursuing some of the big touchscreen display manufacturers,’ she adds.
Positive twist
Back in London, Shaffer’s team has further developed the idea to replace the alkali metal reduction step with an electrochemical process. ‘In principle, all you need to do is add charge,’ says Shaffer. After working out that they could electrochemically make nanotubide, the natural progression was to try and make a positively charged ‘nanotubium’ as well.2 ‘That opens up a whole different branch of chemistry for nanotubes that hasn’t been explored yet,’ says Shaffer.
Linde has licensed patents for both the ammonia–metal process and electrochemical methods, Fogden confirms. ‘At the moment we’re focusing on the ammonia route because that’s the furthest along,’ she says.












No comments yet