Helicene electrocatalysts offer a metal-free way to convert carbon dioxide into valuable chemicals. The catalysts drive the process up to 1000 times faster than other organic compounds and represent ‘the first example of a true organic electrocatalyst for carbon dioxide reduction’, according to the researchers who developed them.
The team led by Joyanta Choudhury at the Indian Institute of Science Education and Research in Bhopal, found inspiration in the way plants convert carbon dioxide into carbohydrates. ‘Our synthetic molecules mimic the NADP/NADPH system, [in terms of] the central pyridine ring structure and function,’ he explains.
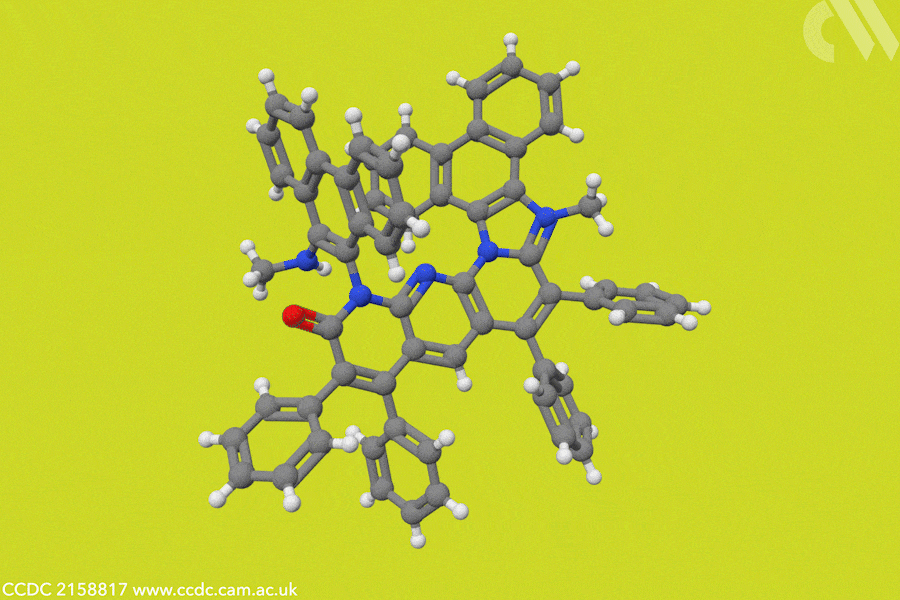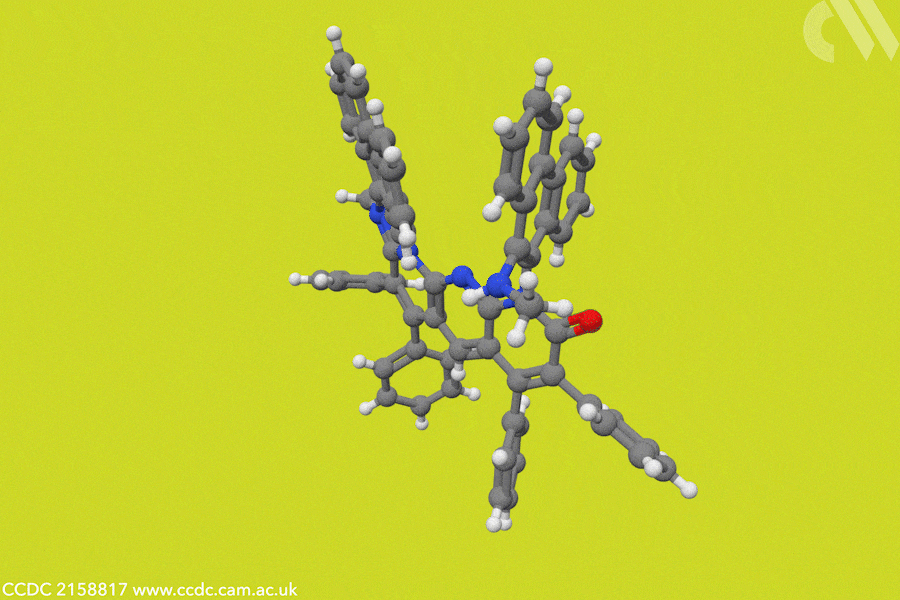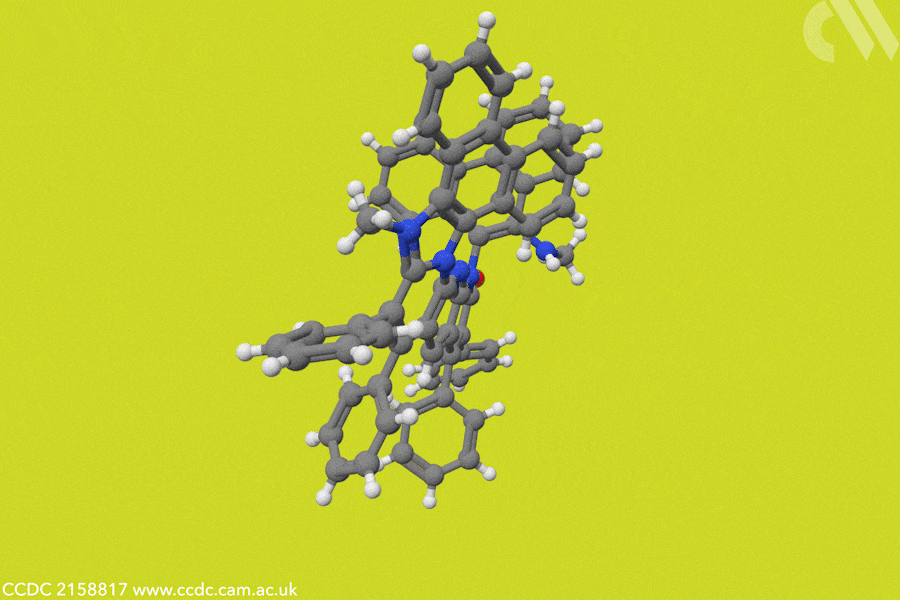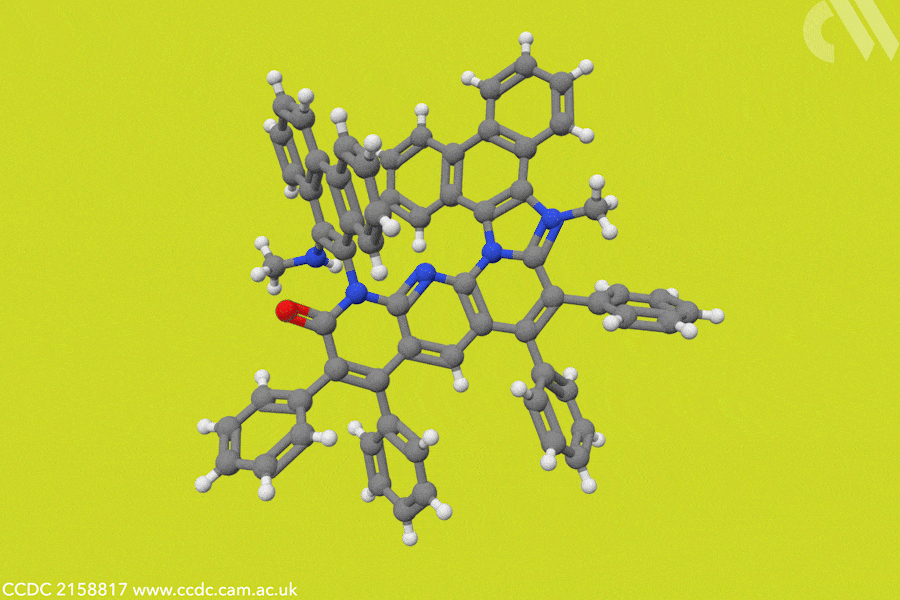
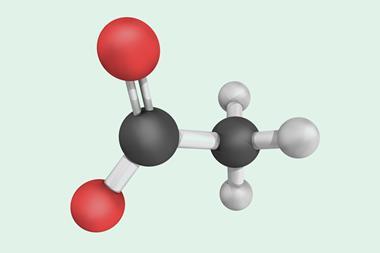
In photosynthesis, NADPH is a cofactor that efficiently transfers hydrides to captured carbon dioxide molecules, a key step in the formation of sugars and biomass. ‘Over the years, organic hydrides have been used in the reduction of substrates like alkenes, imines, and carbonyl products,’ adds Choudhury. In this case, Choudhury’s team designed a hydride donor based on a helicene structure, to create an artificial NADP analogue that drives the electrocatalytic conversion of carbon dioxide into formate ions.
These organic analogues offer several advantages, among them stability and tuneability. Because of their simpler structures, NADP analogues are also easily accessible in the lab, says Choudhury. ‘Moreover, structural modifications [allow us] to tune their reactivity at wish,’ he adds.
‘It features [up to] 1000-fold enhancement of the existing turnover values for similar organic compounds’
Joyanta Choudhury, Indian Institute of Science Education and Research, Bhopal
The helicenes are prepared in a one-pot reaction from simple starting materials. Most importantly, the team has reduced the amount of helicenes used in the electrochemical conversion of carbon dioxide to around 1% – which is considered a catalytic quantity. ‘It features [up to] 1000-fold enhancement of the existing turnover values for similar organic compounds,’ adds Choudhury.
‘It’s a significant improvement, and proof of a catalytic system,’ says Carla Casadevall, an expert in artificial photosynthesis at the University Rovira i Virgili and ICIQ in Tarragona, Spain. ‘Apart from offering a metal-free alternative, [helicenes] regenerate electrochemically following a bio-inspired proton-coupled electron-transfer,’ she says. It’s this process that allows the unusual boost in activity observed. ‘The rational structural design of the helicenes improved its stability … and consequently [that of] the electrochemically generated intermediates,’ she adds.
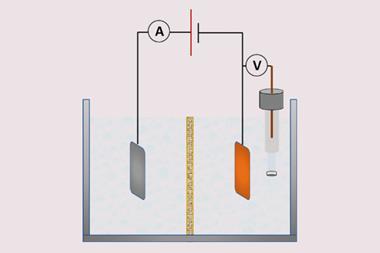
Although the catalytic process is metal-free, some of the demonstrations required electrodes based on hazardous elements, including mercury. ‘Nevertheless, the authors have also proven the reaction works with simple glassy carbon electrodes,’ explains Casadevall. This technology is widely used in electrochemistry and had previously been successful in carbon reduction experiments.
According to Casadevall, the next challenges will be to improve the efficiency – decreasing the overpotential of the electrocatalytic carbon dioxide reduction – and recyclability of the system.
‘The high overpotential and the stability for long-term electrolysis are still issues to address,’ acknowledges Choudhury. Currently, the team is attempting to solve these issues by modifying the backbone of the helicene catalyst and adjusting the reaction conditions. Choudhury also notes that the team is exploring applications of helicenes in catalysis beyond electrochemistry, including a photochemical strategy for carbon dioxide reduction.
References
P Karak et al, J. Am. Chem. Soc., 2023, 145, 13, 7230, DOI: 10.1021/jacs.2c12883










1 Reader's comment