Scientists have used a foldamer helix as a supramolecular aide to compartmentalise a rotaxane and displace its macrocycle to a site for which it has no real affinity. The product resulting from subsequent cleavage is an otherwise unlikely [2]rotaxane.1
Rotaxanes are mechanically-interlocked molecules, in which a macrocycle encircles an axle. The axle ends are capped with bulky groups, ensuring a mechanical bond. Although not covalently linked, the components cannot separate without at least one covalent bond breaking.
The interlocked components in rotaxanes can move with respect to each other – a feature exploited in molecular shuttles. In these systems, co-conformational changes are typically driven by external stimuli, which alter axle–macrocycle interactions. By contrast, examples where an intermolecular recognition of a neutral compound promotes macrocycle translocation are scarce.
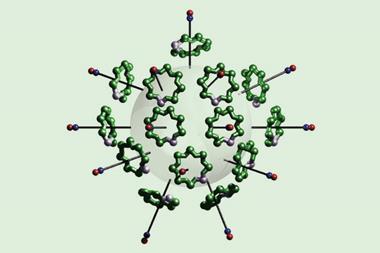
In 2021, a team surrounding Yann Ferrand, from the University of Bordeaux, France, and Frédéric Coutrot, from the University of Montpellier, France, reported foldarotaxanes, where the axle was also encircled by a foldamer helix.2 They showed that macrocycle position influences the association between the helix and the axle, and also that the helix can displace the macrocycle from its preferred site by compartmentalising the axle.
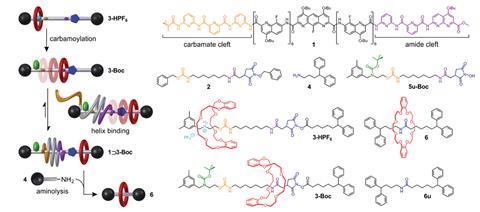
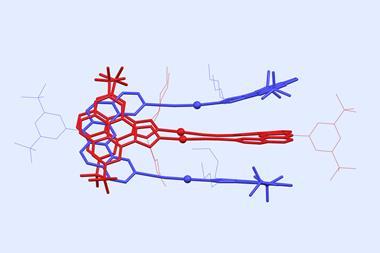
Now, another team surrounding Coutrot and Ferrand has used foldamer-mediated compartmentalisation of a rotaxane shuttle to prepare an improbable [2]rotaxane. Initially, the shuttle contained an ammonium station with high affinity for the macrocycle, and an amide station with lower affinity. Concealing the ammonium through deprotonation-then-carbamoylation induced macrocycle relocation to the amide site. The amide together with a carbamate site served as an axle recognition motif for the foldamer. Therefore, the subsequent binding of the foldamer helix resulted in macrocycle translocation over the cleavable N-hydroxysuccinimide ester, to the unhindered side. Finally, aminolysis-mediated cleavage of the ester produced the improbable [2]rotaxane in 87% yield.
This study is unique because it demonstrates that a foldamer-mediated intermolecular event can serve as stimulus for displacing a macrocycle to a site for which it has no formal affinity. The work paves the way to molecular shuttles and pumps, in which the interdependent motion of a macrocycle and foldamer on a shared axle forms the basis of actuation.
References
1 V Koehler et al, Chem. Commun., 2022, DOI: 10.1039/d2cc03066g
2 M Gauthier et al, Angew. Chem. Int. Ed., 2021, DOI: 10.1002/anie.202100349







No comments yet