Lasers could revolutionise nitrogen fixation, offering a new way to synthesise ammonia under ambient conditions. For the first time, researchers have used commercial carbon dioxide lasers to break the nitrogen–nitrogen triple bond, offering a new green alternative to the Haber–Bosch process.
The international team of researchers used lasers to convert lithium oxide into metallic lithium, which spontaneously reacts with nitrogen in air to form lithium nitride. This salt is easily hydrolysed into ammonia, breaking all current records in terms of yield.
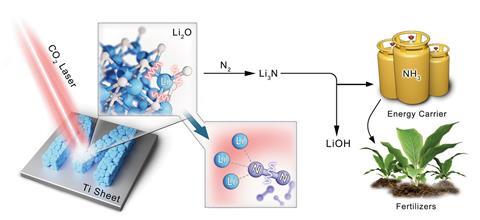
‘We have introduced a pioneering concept, [which] harnesses high-energy lasers to facilitate the conversion of various oxides into nitrides,’ says first author Huize Wang, from the Helmholtz Institute for Renewable Energy, in Germany. ‘We have achieved an unprecedented yield […] under room temperature and atmospheric pressure conditions, notable when compared to other methods,’ he adds. The yield is two orders of magnitude higher than other state-of-the-art solutions, including electrochemical and mechanochemical methods.
‘It’s a new method for the production of green ammonia,’ says Victor Mougel, an expert in the electrochemical transformation of small molecules based at ETH Zurich, Switzerland. ‘[Alternative] methods are potentially more sustainable than the Haber–Bosch process, which is very energy intensive as it operates at high temperature and pressure and […] contributes to carbon dioxide emissions.’ As the process works in ambient conditions it ‘offers operational flexibility, as well as the environmental benefits’. This process could also allow ammonia to be produced where it is needed, cutting the cost of transportation.
The team generated metallic lithium from lithium oxide, thanks to an infrared laser that provides enough energy to dissociate the lithium–oxygen bonds. When exposed to air, metallic lithium spontaneously binds nitrogen breaking the nitrogen–nitrogen triple covalent bond and generating lithium nitride. ‘[We then] hydrolyse this laser-generated lithium nitride to obtain ammonia gas and lithium hydroxide,’ explains Wang. Moreover, this approach offers the opportunity for chemical cycling. ‘A laser [can] induce the conversion of lithium hydroxide back into lithium nitride, effectively closing the lithium cycle,’ he adds. ‘This [is] also another novel concept – the conversion from hydroxide to nitride.’
Ifan Stephens, an expert in electrochemistry and nitrogen fixation at Imperial College London, UK, is still sceptical. ‘I’m not certain [these] high rates can be sustained for long periods of time,’ he says. ‘Moreover, […] the fact that it is a batch process, as opposed to a continuous process, would pose significant limitations to its viability.’ In contrast, electrochemical technologies work continuously, which ‘offers a significant advantage over the new laser-induced method’, according to Stephens. Additionally, the energy needs of the lasers could pose problems for scaling-up ammonia synthesis. ‘If you […] make ammonia on a small scale, as a fertiliser for remote locations, then the energy efficiency becomes less important,’ he adds.
‘Compared to electrochemistry, our method offers significant advantages [such as] desolvation and simplification,’ argues Wang. Plus, ‘the scale-up […] presents the most significant challenge for all emerging approaches for ammonia synthesis’. The researchers envision scaling up the process by distributing lithium oxide powder on a gridded surface, then irradiating the arrays of reaction cells with the laser, sequentially. Additionally, researchers have observed similar behaviour with other oxides, such as magnesium, aluminium, zinc and calcium – although the yield is lower. ‘[It] could be because these other oxides are more difficult to dissociate and hydrolyse,’ explains Stephens. However, the reactivity of alkaline and alkaline earth metals towards nitrogen seems promising. ‘Our recent work shows that more abundant metals, such as magnesium and calcium can also dissociate nitrogen,’ he says.
References
H Wang et al, Nat. Commun., 2023, 14, 5668, (DOI: 10.1038/s41467-023-41441-0)















1 Reader's comment