Cobalt–copper system works in water and tolerates air
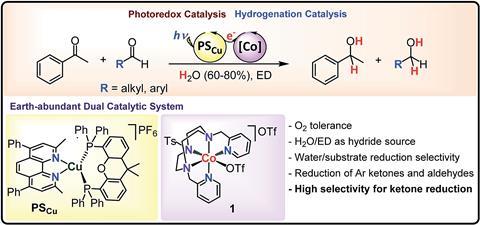
Researchers in Spain have developed a light-driven catalytic process that offers a greener way to produce organic alcohols – important compounds used to manufacture pharmaceuticals and pesticides.
The new technique, developed by Julio Lloret-Fillol’s team at the Institute of Chemical Research of Catalonia (ICIQ), is air tolerant, works in water, and doesn’t use the rare-earth metals often called upon to catalyse such reactions. Instead, it reduces aldehydes and ketones using a system that combines cobalt and copper. A cobalt complex plays an essential role in promoting the reaction, while a copper co-catalyst significantly increases the system’s activity. Visible light activates the process at temperatures as low as 15°C. The procedure’s tolerance to air is unusual, as oxygen normally deactivates catalysts based on earth-abundant elements.
The team demonstrated the reaction with more than 20 aryl ketone substrates, as well as a selection of both aromatic and aliphatic aldehydes. And although they tested the catalytic system in water, it showed a selectivity in favour of reducing the organic substrates over the water molecules of more than 2000:1. Surprisingly, the technique also preferentially reduces acetophenone over highly reactive aliphatic aldehydes when applied to mixtures of substrates.









No comments yet