Advanced imaging has revealed how a membrane protein’s unique structure enables a bacterial predator to attack and consume other microbes. The researchers behind the work say that the findings could inspire new ways to target harmful bacteria, bringing potential applications in medicine and biotechnology.
The new research focuses on PopA, a porin-like protein found in the outer membrane of Bdellovibrio bacteriovorus (B. bacteriovorus) – a predatory microbe that targets a wide range of other Gram-negative bacteria. Unusually, PopA can move between the outer membrane of one cell and into the inner membrane of another, explains Andrew Lovering, a structural biologist at the University of Birmingham in the UK, who led the project.
‘This goes against all conventional rules of how membrane proteins are put into membranes and what shape they take,’ he explains.
Lovering’s PhD student Rebecca Parr started the work on PopA just before the Covid-19 pandemic.
‘This was in the era before AlphaFold prediction was as confident – so we had lots of questions: what do you look like? Why do you look like that? Does that help you exist both in inner membranes and outer membranes,’ says Lovering.
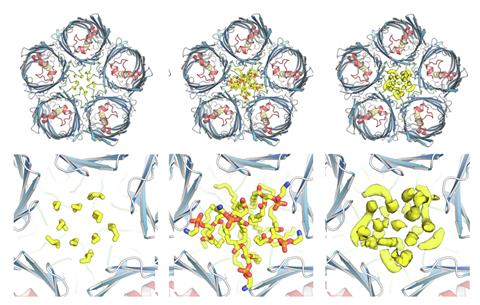
Using x-ray crystallography and cryo-electron microscopy, Parr discovered that the protein formed a unique fivefold structure, unlike the usual single or three-part structures seen in other outer membrane proteins.
At first, Lovering was sceptical when Parr told him what she’d found. ‘I said you’re probably a bit mistaken, because these things come in threes, but she said: “No, no, it’s definitely five.”’ he recalls. ‘As the imaging improved and the map improved, we were like: “Whoa – this is totally different!”’
It’s a trap!
To find out more about the function of PopA, they introduced it to Escherichia coli (E. coli) bacteria where it appeared to cause significant defects in cells’ outer and inner membranes. This suggested that PopA may play in a role in how B. bacteriovorus attacks and consumes other bacteria.
The team also discovered that the PopA protein has a bowl-like shape in which it can capture lipid molecules. ‘Because this traps the lipid inside … you’re messing with the ratios of lipid in the membrane,’ explains Lovering. ‘Most membrane proteins sit in this cloud of lipopolysaccarides … whereas [PopA] just clears them out the way.’
Further analysis uncovered homologues of PopA across a variety of bacterial species that form tetramers, hexamers and sometimes even nonamers, and all had the same lipid-trapping feature.
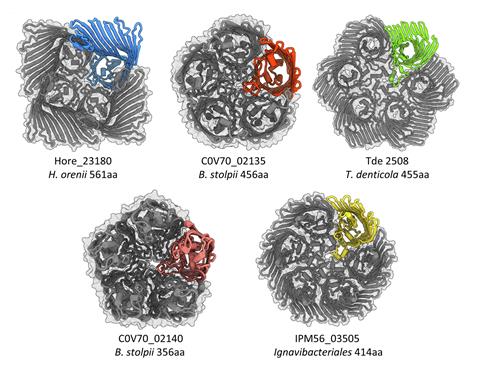
Lovering says that research on outer membrane proteins has been dominated by E. coli and Pseudomonas, leading to a ‘restrictive view’. Finding ‘rulebreakers’ like PopA in other species gives scientists a deeper understanding on what these biomolecules are capable of, he notes.
‘So if you’re interested in the wider biology, you’re not shackled by the textbook view,’ he says. ‘This is [one example] – as soon as we start getting a few, we can change our expectation of what membrane proteins can do.’
Mohammed Kaplan, a microbiology expert at the University of Chicago, US, who was not involved in the project, is intrigued by the findings.
‘The interesting thing is this protein is found in a small predator, which captures other Gram-negative bacteria and consumes them. This protein goes from the outer membrane of this predatory bacteria to the inner membrane of the prey cell, and so it plays a big role in predation,’ he says. ‘Understanding this predation process is a very important thing … [because] it has repercussions in understanding fundamental biological questions related to evolution and cell–cell interaction.’
However, Kaplan notes that the findings raise new questions for microbiologists. ‘One important question, which [Lovering’s team] also highlights, is how does PopA go from the predator to the prey? Is it secreted through an outer membrane vesicle or by another way? That’s still unknown.’
References
R J Parr, et al, Nat. Commun., 2025, 16, 6213 (DOI: 10.1038/s41467-025-61633-0)

















No comments yet