Researchers use metal ions to guide synthesis of 'double-threaded' [3]rotaxane
Chemists in France have developed a simple method to synthesise tricky ’[3]rotaxane’ molecules for potential applications in intelligent materials and molecular machines. The method, which uses metal ions as a template, should be able to provide high yields of [3]rotaxane and other threaded species.
Rotaxanes are a class of compound that contain dumbbell-shaped molecules threaded through other, ring-shaped molecules. They have generated much interest over the past two decades because they can change shape in response to an external stimulus, an ability that lends them to intelligent materials and molecular machines in which the molecules themselves process information.
In general the number of components provides the name, so that an assembly with just one dumbbell and one ring molecule is called a [2]rotaxane. A [3]rotaxane could therefore contain either one dumbbell and two rings, or two dumbbells and one ring. However, the latter ’double threaded’ assembly, which is more complex, has proved very difficult to create.
Jean-Pierre Sauvage and Alexander Prikhod’ko at the Louis Pasteur University in Strasbourg, France, have found that metal ions can make the synthesis of these double-threaded [3]rotaxanes straightforward. They take ring and string molecules and introduce cobalt (II) ions, which bonds to the molecules in such a way that two strings both thread through a single ring. In the next step they perform a ’stoppering reaction’ to add large molecular groups to the ends of the strings, thereby making them dumbbell-shaped and preventing the rings from slipping off. In the final step the central metal ions are reacted away, leaving pure, double-threaded [3]rotaxane molecules.
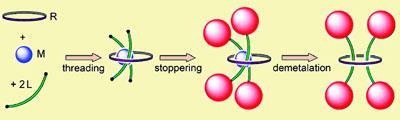
Prikhod’ko said the ’beauty’ of the synthesis was in getting the right precursor molecules and ions that would work together. ’As a matter of fact, it took several years for Sauvage’s group to find the steps to a [3]rotaxane molecule,’ he added.
Fraser Stoddart, a chemist who has performed extensive research on rotaxanes, described the research as ’exquisitely conceived and beautifully executed’ and lauds the lifetime achievements of Sauvage, who will celebrate his 65th birthday this year. ’Chemical synthesis today does not come much better than this latest one from the Sauvage stable,’ he added. ’The research described in this full paper is ingenious. The results are spectacular. I don’t know if it’s important but I would say it is highly significant.’
Jon Cartwright
References
et alJACS, 2009. DOI: 10.1021/ja809267z






No comments yet