The iron atom at the centre of ferrocene has been oxidised for the first time. A metal–organic framework (MOF) facilitates the transformation, which converts ferrocene into a stretched, high-spin state that can accept oxygen from the air.
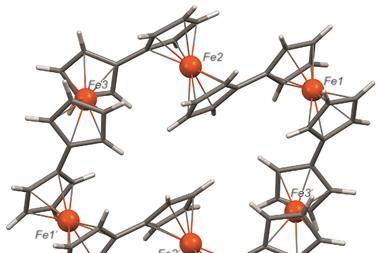
Ferrocene contains an iron atom sandwiched between two cyclopentadiene rings. Ever since it was first made over 70 years ago, chemists have unpicked the organometallic molecule’s distinct reactivity, thermal and chemical stability, steric properties and redox activity. However, scientists have never managed to oxidise ferrocene’s iron centre – until now.
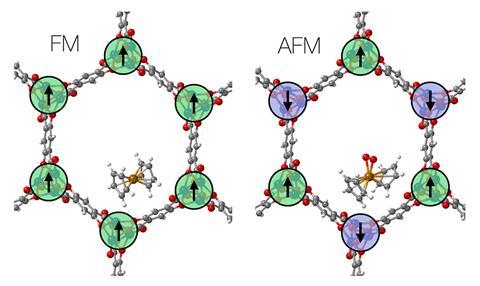
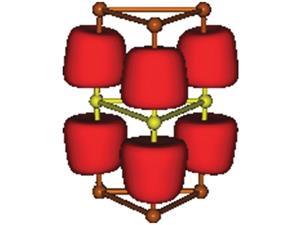
Timo Thonhauser, from Wake Forest University in the US, and colleagues devised a cobalt-based MOF to overcome ferrocene’s unreactive iron centre. The cobalt atoms form chains that can either be ferromagnetic or anti-ferromagnetic in nature. Adsorbing ferrocene into an anti-ferromagnetic chain sees the sandwich compound’s structure bend, which weakens the ligand field energy of the cyclopentadiene rings and allows iron to take on a high-spin state.
Oxygen in the air oxidises this high-spin iron, pushing the cyclopentadiene ligands apart to give ferrocene a more angular shape. This structure is distinct to the commonly known sandwich complex motif.
The work extends scientists’ understanding surrounding the electronic and magnetic structure of ferrocene so could feed into research developing oxygen storage systems and artificial blood.
References
S Ullah et al, J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c05754












No comments yet