Lithium and sodium become insulators and semiconductors in diamond anvil cells
Two independent teams have demonstrated that metals can lose their high conductivity under pressure, proving that the standard models used to describe their behaviour are inadequate.
Yanming Ma of Jilin University in Changchun, China, and colleagues from Switzerland, Germany, Russia and the US have shown that sufficient pressure causes sodium to become optically transparent. Meanwhile, Takahiro Matsuoka and Katsuya Shimizu of Osaka University, Japan, have shown that it causes lithium to turn into a semiconductor.
Pressure causes atoms in a material to squeeze closer together. In insulators, the process reduces the gap between the valance and conduction bands, sometimes so much that two bands overlap and allow electrical conductivity. But although metals have previously demonstrated odd characteristics at high pressure, the absolute reverse transition - one from a metal to insulator - has never been seen.
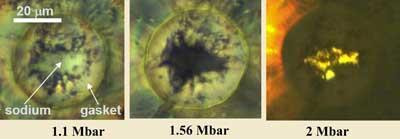
Ma’s group squeezed a micrometer-sized sample of sodium between two diamonds in an anvil and used various techniques, including optical absorption, Raman spectroscopy and micro X-ray diffraction, to observe its behaviour. At a pressure of 130GPa the sodium changed from its usual colour of white to black, and at 200GPa - roughly half the pressure at the centre of the Earth - it turned optically transparent. This latter transition means it must have developed a bandgap of about 3eV, somewhere above that of a semiconductor and below that of a pure insulator.
Matsuoka and Shimizu have developed a special gasket that enabled them to take electrical resistance measurements at high pressures in an anvil cell. At 80GPa, they found the conductivity of their lithium sample dropped to 0.0001 of its value at atmospheric pressure. With this lack of conductivity, lithium is more like a semiconductor.
Both sodium and lithium are ’simple’ metals, which means their electrical behaviour can usually be accurately described by the so-called free-electron model. In this model, the outermost electrons of atoms move through the structure easily like a dense gas. Now, the observed transition to insulators or semiconductors proves that the free-electron model has its limitations. Matsuoka and Shimizu do not know what structure their pressurised lithium takes, but Ma and colleagues’ additional spectroscopy techniques have given them an insight. They believe their sodium sample takes on a new type of ’ionic’ structure - what they call a highly distorted double-hexagonal close-packed structure - in which the outermost electrons are forced into localised interstitial spaces between atoms.
’Their picture of transparent sodium is really beautiful,’ says Matsuoka of Ma and colleagues’ study. ’Though their work is not based on resistance measurements, their findings strongly indicate sodium transits to a semiconductor-insulator in a dense state.’
Ma says it is unfortunate that Matsuoka and Shimizu could not solve the crystal structure of semiconducting lithium, but notes that the Japanese researchers’ work supports their findings. ’Further fine experiments are needed in the future,’ he adds.
Jon Cartwright
References
Y Ma et al., Nature, 2009, DOI:10.1038/nature07786
T Matsuoka and K Shimizu, Nature, 2009, DOI:10.1038/nature07827






No comments yet