Iodine(iii) and iodine(v) coexist in new nitroiodoxybenzene-based oxidant

Chemists have made the first hypervalent iodine compound containing both iodine(iii) and iodine(v). The new oxidant has proved effective in making 2-unsubstituted 2H-azirines from enamines, a synthesis not possible using existing hypervalent iodine reagents.
Hypervalent iodine compounds are proving to be less toxic and more useful for step-efficient syntheses than traditional oxidants. Current hypervalent iodine compounds contain either iodine(iii) or iodine(v). Now, a team led by Yunfei Du from Tianjin University in China has created a new hypervalent iodine reagent with an unusual dual iodine(iii/v) structure.
Preparing the new oxidant involved reacting o-nitroiodobenzene with m-chloroperoxybenzoic acid in acetic acid. Instead of the expected product, o -nitroiodoxybenzene, an orange solid formed. Single-crystal x-ray crystallography allowed the team to establish that the orange solid contained both iodine(iii) and iodine(v), making it the first mixed valence number hypervalent iodine compound. Interactions between the iodine atoms and electron-withdrawing nitro group at the ortho phenyl position appear to stabilise the structure.
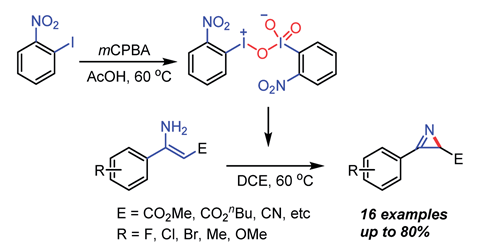
Traditional hypervalent iodine reagents cannot oxidise enamines to produce 2-unsubstituted 2H-azirines but the new mixed valence oxidant can and the team are optimistic it will have other applications.













No comments yet