A metal-organic framework has been created that could trap radioactive isotopes of iodine produced by nuclear power plants
A porous material that can hold up to one and a quarter times its own weight of molecular iodine could help to mop up gaseous radioactive isotopes of the element. A US-based team has shown that the material could be useful for trapping the radioactive iodine released during nuclear fuel reprocessing, and also help to prevent inadvertent environmental release of the gas, as occurred earlier this year at Japan’s Fukushima nuclear power plants.
The material in question is a metal-organic framework (MOF), a highly porous structure with a huge internal surface area ideal for adsorbing large volumes of gas. Tina Nenoff at Sandia National Laboratories in Albuquerque, New Mexico, and her colleagues have shown that a MOF known as zeolitic imidazolate framework-8 (ZIF-8) can be used to permanently capture large volumes of iodine.
Radioactive iodine isotopes are one of the more problematic products of nuclear fission to deal with, because they form a highly mobile volatile gas. 129I has a half-life of 1.57 x 107 years, while 131I has a half-life of 8 days. The materials currently used to trap radioactive iodine can only absorb a limited volume of the gas, says Nenoff.
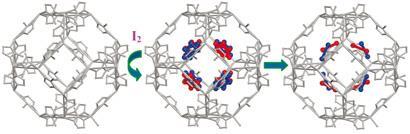
Nenoff has now shown that ZIF-8 can hold 125 per cent by weight of iodine, which is an order of magnitude higher than the zeolites currently used. ZIF-8 was chosen by the team because its pore size of 3.4? is just large enough to let iodine diffuse in. Once inside the ZIF cage, the iodine is trapped unless the material is heated above 575K.
The ability to gently heat the material without losing any iodine is essential - it means that the team have been able to incorporate it into a low-temperature sintering glass, permanently immobilising the iodine within a homogenous glass that could be sent for long-term storage.
To assess its potential for real-world application, Nenoff has sent ZIF-8 to be tested by colleagues at Oak Ridge National Lab in Tennessee, US, where it will be assessed as a molecular iodine adsorbent during nuclear fuel reprocessing.
’We’re also taking the information that we know, which includes the chemical bonding inside the pore, and the pore structure, and designing new ligands to make new MOFs. We’re just getting ready to publish on those,’ she says.
The work is a useful development, says Lou Vance, a chief research scientist in materials at the Australian Nuclear Science and Technology Organisation in Sydney. ’The material seems to have a good propensity to pick up the gas - the volume that they can capture is really pretty good,’ he says. The material would be particularly good for trapping the short-lived 131I for the few months it takes to decay into the stable 131Xe, he adds - although in comparison with
immobilising high level waste as a whole, immobilising iodine is a relatively minor problem.
James Mitchell Crow
References
D F Sava et al, J. Am. Chem. Soc., 2011, DOI: 10.1021/ja204757x






No comments yet