Colorimetric tests could find applications in NMR, photoacoustics and quality control
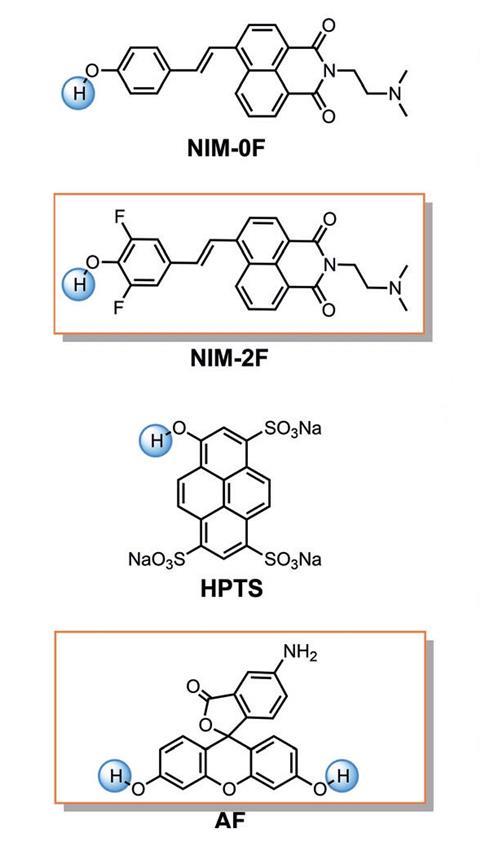
Researchers have developed a new way to distinguish water from deuterium oxide (D2O), using simple organic molecules as colour-changing optical sensors. Heavy water has a slightly higher pH than water, and chemists at Sichuan University in China took advantage of this, identifying a set of purely organic chromophores that can detect the subtle difference.1
‘Deuterium oxide is a common deuterated solvent in our laboratory,’ explains Xujun Zheng, who led the research team together with Zhiyun Lu. ‘Through NMR experiments, we observed that, due to water contamination, the purity of D2O often changed in different batches. This triggered our interest in developing a more facile method to determine the purity of heavy water.’
The group, which had previously worked on similar organic optical sensors to detect fluoride ions,2 decided to look for molecules with the exact acidity values to display different optical responses when exposed to water and deuterium oxide. ‘The difference in the pH of water and heavy water is known, but it is great to see how this property has now been used to develop new optical sensors,’ says Alice Soldà, an expert in functional imaging techniques at the Technical University of Munich, Germany, who was not involved in the research. Soldà is also impressed at the sensors’ sensitivity: ‘[It] is quite remarkable. They are able to detect [parts per million] of heavy water.’
In the presence of heavy water, a hydroxyl group in the organic sensors deprotonates, yielding an anionic species with a completely different colour. Some of the molecules studied produce an obvious colour change that can be noticed directly under visible light. In other cases, ultraviolet light is needed to spot the difference. ‘But this is still quite easy, you would just need put your vial under the UV lamp,’ explains Soldà. ‘The authors have done a very thorough job characterising the different chromophores and their spectral properties at different percentage of heavy water,’ she adds.
The new technique is also cheaper, quicker, easy to handle, and more environmentally friendly than existing ones, which usually require hard-to-operate machinery and specialised technicians, or use sensors based on scarce elements like indium, gallium or lanthanoids. ‘Our sensors can be tailored through rational design, and their properties can be more easily optimised to our needs,’ says Lu. ‘Although some of our solutions are confined to small-scale laboratory synthesis, one of our probes is based on 5-aminofluorescein, which can be purchased with relatively low cost.’ Soldà agrees: ‘Organic sensors are indeed cheaper, and the readout is very quick and easy to understand.’
As well as being used to test the quality of heavy water for NMR experiments, these deuterium oxide sensors could also find applications in photoacoustics, a non-invasive imaging technique becoming increasingly popular in medical diagnostics, which uses heavy water as a ‘contrast’ because of its higher mass. And as these quick probes are ‘quite convenient, […] they may easily find applications in the quality control processes of heavy water plants,’ says Lu.
References
- Y Luo et al. Angew. Chem. Int. Ed. 2019, DOI: 10.1002/anie.201900806
- X Zheng et al. ACS Appl. Mater. Interfaces 2014, 6, 7996, DOI: 10.1021/am501546h















No comments yet