New pathways for controlled synthesis of fluorinated targets have been opened up by UK researchers.
New pathways for controlled synthesis of fluorinated targets have been opened up by the fluorination of organosilanes with the silyl group directly attached to or adjacent to an aryl or alkenyl group.
Fluorine-containing organic materials are commonly used in materials, medicinal, pharmaceutical, and agrochemical science. Naturally occurring organofluorine compounds are rare and their reactivity is unusual.
V?ronique Gouverneur and Matthew Tredwell at the University of Oxford, UK, have developed more versatile routes to fluorine-containing molecules including those with a chiral centre.
These can be made by fluorinating organosilanes where the silyl group is directly attached to or adjacent to an aryl or alkenyl group. This creates new possibilities for the stereoselective synthesis of fluorinated targets.
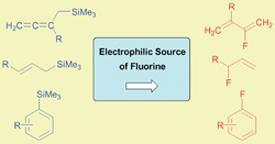
Studies to confirm the reaction mechanisms associated with these reactions are the next step in establishing the utility of silanes in the synthesis of organofluorines.
Suzanne J Abbott
References
M Tredwell and V Gouverneur, Org. Biomol. Chem., 2006, 4, 26 (DOI: 10.1039/<MAN>b513399h</MAN>)






No comments yet