Chemists are becoming ever more clever in their attempts to deliver new genes to particular cells, bringing the prospect of gene therapy a step closer. Maria Burke reports
Chemists are becoming ever more clever in their attempts to deliver new genes to particular cells, bringing the prospect of gene therapy a step closer. Maria Burke reports
Gene therapy has been talked about for more than 20 years. It sounds simple: deliver useful genes to the right spot, and turn them off and on. Hailed first as potential treatments for hereditary diseases such as cystic fibrosis, gene therapy could also help to treat and prevent infectious diseases such as Aids and cancer, as well as cardiovascular disease.
The first successful gene therapy on humans was performed in 1990 by researchers W. French Anderson, Michael Blaese and Ken Culver of the US National Institutes of Health. A four-year-old child was treated for adenosine deaminase deficiency, a rare genetic disease in which children are born with severe immunodeficiency and are prone to repeated serious infections.
Following this success, the field developed steadily until a setback in 1999 when a teenage volunteer died in a gene therapy clinical trial at the University of Pennsylvania. His immune system, it was thought, had over-reacted to the virus used to carry the gene into the tissue. Thirteen years after its first success, a gene therapy still has not been approved for clinical use.
Viruses such as adenovirus and retroviruses have been a popular choice for a gene carrier, or vector. They home in on specific tissues, invade cells and manipulate cell machinery to produce specific proteins. But there are disadvantages with using viruses as vectors. There are lingering concerns about associations with human disease and they can cause an immune response, making repeated administrations of the genetic material ineffective.
For the past decade, researchers have increasingly been turning their attention to nonviral vectors. The two main approaches involve combining DNA with cationic lipids (lipoplexes) and/or cationic polymers (polyplexes. Cationic lipids and polymers self-assemble with DNA, which is a polyanion, to form small particles. They condense the DNA, making it easier to get inside the cells. Significantly, they can fly under the radar of the body’s immune system and can carry more DNA than viruses, allowing them to deliver larger genes. They are also easier and cheaper to make.
Non-viral approaches are based more on basic science - the ’bottom-up’ approach - rather than viruses which take a ’top-down’ approach, says Len Seymour of Oxford University’s clinical pharmacology department. ’[Non-virals] are likely to be more stable, easier to scale up and produce, and meet better patient compliance. They are likely to be preferred ultimately but there are many stages of the process that need to be improved’.
However, there are several key problems before non-viral systems could be used effectively in in vivo applications. One is stability. To be useful therapeutically, the lipoplexes or polyplexes must be administered in high concentrations. But as their concentration increases, these positively charged particles clump together. To remain as discrete particles in the blood, they have to be stabilised. One solution is to use hydrophilic polymers like polyethylene glycol to provide steric stabilisation.
Although this can affect the particle’s ability to condense DNA, scientists have managed to make highly concentrated, spherical particles. What’s more, particles must be stable enough to reach their destination but be able to ’disassemble’ and release their DNA cargo once they are inside a cell.
A second limitation of non-viral systems is their toxicity and much current work is focused on making carriers with lower toxicity. Recent evidence is pointing to significant advantages in using preparations of relatively low molecular weight polycations, such as chitosan or cyclodextrin-containing polymers, both in cultured cells and animals.
As well as showing low toxicity, the systems must interact as little as possible with proteins and cell surfaces on their journey through the blood to the target cells. Scientists are trying to direct the complexes so they pass straight to their goal. Attaching ligands that interact only with specific surface receptors is one way of doing this. Examples are small molecules such as folate and galactose, or peptides and proteins such as transferrin.
Non-viral systems are still not as efficient as viruses at shuttling genes into tissues so scientists are working on penetrating a higher percentage of cells in target tissues. Once inside the right tissue, the particle must enter the cell and then enter the nucleus for gene expression to take place. Since nuclear pores are 25-50nm wide, bulky polyplexes and lipoplexes can only enter the nucleus at certain times, such as during nuclear membrane breakdown or cell division. However, delivering DNA to the nucleus of non-dividing cells like neurones is extremely difficult. Most scientists believe that without some means of assisted transport to and into the nucleus, non-viral gene delivery is likely to remain inefficient. Linear cations, nuclear localisation signal peptides or particular targeting ligands are all under investigation.
A further hurdle is to prolong expression of the imported gene once inside the cells. All non-viral systems could benefit from increasing levels of expression in any cell types, comments Mark Davis of the California Institute of Technology’s chemical engineering department. ’However, the expression levels are, in some cases, likely to be sufficient to prove therapeutic, especially in light of being able to administer repeated doses’.
Len Seymour and his team at Oxford University are concentrating on all these issues in designing a multifunctional self-assembling vector based on complex polymers. ’Early studies are very promising, and we are gradually increasing the technological complexity of the vector to enable it to address the demands of the complex biological environment.’
Using polycations such as poly(L-lysine) or poly(ethylene imine), Seymour is working with 100nm-wide polyplexes. Wrapped around their surfaces are multivalent reactive polymers - poly[N-(2-hydroxypropyl)meth- acrylamide] (pHPMA). These polymers stop the polyplexes falling apart after intravenous injection, by combining stabilisation with a strengthened outer layer. ’Results using this approach have been excellent’, he reports.
Seymour has designed his vector to be activated ready for transcription (the first stage of protein production) once at its target site. By adding polymers based on disulphide bonds to the outer coating, he has found that the polyplex is rapidly reduced once inside cells. ’This trigger mechanism enables us now to build vectors that combine good delivery properties with efficient transcription following cell entry, providing exactly the sort of vector we require for targeted expression of therapeutic transgenes’.
To enhance nuclear uptake, and also increase gene expression, Seymour is experimenting with ’nuclear localisation sequences’ (NLS), specific sequences of amino acids that are found on nucleoproteins. He has found that attaching NLS sequences to linear DNA improves entry through the nuclear membrane pores.
Another way to get the DNA through the nuclear pores is being investigated by researchers at Case Western University and Copernicus Therapeutics in Ohio. They have developed a way to pack DNA into particles 25nm across using a compacting polymer consisting essentially of a polyethylene glycol-substituted polylysine. ’For typical expression plasmids, the nanoparticles are less than 25nm in diameter if compacting to form a sphere, or rods that have a length of 75-125nm with a diameter of 12-15nm’, explains Mark Cooper, senior vice-president, science and medical affairs, at Copernicus. ’The small size of these nanoparticles permits robust gene transfer in non-dividing cells - a unique capability for a non-viral gene transfer system’.
So far, Cooper reports, the data indicate that the complexes are not toxic and evade the immune system. He adds: ’The ability of the Copernicus DNA nanoparticles to robustly transfect non-dividing cells greatly increases their efficiency and represents a major advance for non-viral vectors’. Cooper believes that the technology will provide products for cystic fibrosis (CF), other genetic disorders such as haemophilia, cancers and vaccines. In fact, the company started phase I/II trials in April 2002 to investigate using these nanoparticles to deliver a normal copy of the CF gene to 12 patients. Gene transfer data were due to become available last month.
Insert Therapeutics in Pasadena, CA, is also working with condensing agents but using a new class of linear polymer containing cyclodextrins created by Mark Davis of Caltech rather than lipids or more traditional polymers. These cup-shaped cyclic oligomers of glucose are biocompatible with humans and animals, and are already approved by the US authorities for oral and intravenous administration. ’The use of the cyclodextrin in the polymer provides for an entirely new way of making targeted particles for systemic application’, Davis says.
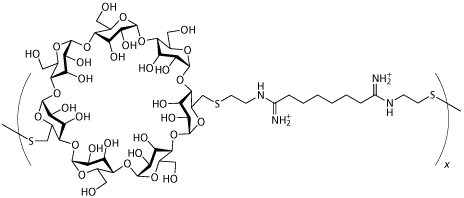
Cyclodextrin polymer delivery system created by Mark Davis |
Scientists at Insert Therapeutics have shown that cyclodextrins can deliver several types of nucleic acids - plasmids (rings of DNA) for example - in small animals and established that these new polymers have very low toxicity.4 Working with Johnson & Johnson, Insert has also demonstrated that the cyclodextrin polymer delivery system can successfully target tumours and deliver nucleic acids from an intravenous injection, reports Davis. ’The system provides a lack of toxicity and it is sufficient to allow multiple dosing and even continuous infusions of doses much higher than have been reported previously’, he says.
Meanwhile, over in Wisconsin, Mirus Corporation claims to be generating some of the first DNA particles that are both negatively charged and stable in the bloodstream. ’In comparison with other non-viral synthetic approaches, we have concentrated on creating particles that are small, negatively charged and stable under physiologic conditions so that they can be delivered via the bloodstream to a target tissue’, claims Jim Hagstrom, vice-president of scientific operations at Mirus. ’We have accomplished these goals and we are now working on ways to allow for better release and transport of the DNA inside the target cell’.
Mirus is developing non-viral DNA-containing particles between 50 and 150nm in size. They use a variety of methods but all involve condensing the DNA by interaction with a cationic molecule, either cationic polymers or cationic lipids.5
In one process called template polymerisation, the DNA itself is the template and serves as the backbone to which cationic molecules bind electrostatically, explains Hagstrom. ’Once bound, the cationic molecules can be polymerised along the DNA template via a step-polymerisation or chain polymerisation process to form much larger cationic polymers. For this process, the cationic molecules - that is, monomers - to be polymerised must contain two positive charges. When small polycations are used with two or three positive charges per monomer, the DNA condensation occurs during the polymerisation process as the cationic polymer grows along the negatively charged DNA template. When larger polycation polymers are used as the monomer, condensation occurs prior to the polymerisation of the cationic monomers.’
Mirus has also developed DNA particle technologies in which the surface charge of the particle can be made negative like viruses. First, Mirus forms DNA-polycation particles and then ’recharges’ them by adding negatively charged polymers to the surface. The company has also developed a modified version of template polymerisation called ’caging’ in which the recharging negatively charged polymers are covalently attached to the positively charged inner polymer layer. ’By using this caging approach we have been able to generate salt and serum stable particles that contain a negative surface charge’, says Hagstrom.
For in vivo applications, negatively charged particles are important for several reasons. Following injection into the bloodstream, a negative surface charge allows the particles to circulate without aggregating or sticking to other cells they encounter. Most bloodstream proteins, eg albumin, are negatively charged.
Conversely, when using DNA-containing particles with a positive surface charge, aggregation of particles is a well recognised problem. Once aggregated, these large aggregates can become stuck in the first capillary bed that they reach. Also, cells in vivo can’t ’internalise’ aggregated complexes as efficiently. In addition cationic particles are very difficult to target to a specific type of cell in vivo because these particles will tend to ’stick’ to the surface of any cell type (blood cells and blood vessel cell walls). This is because the plasma membranes of all cells are negatively charged.
The problems of using cationic particles are limited to in vivo applications. For delivery of genes to cells in a culture dish (in vitro), cationic particles (even aggregated ones) can be used efficiently.
Mirus also uses various cationic lipids to condense DNA into small particles as well as various combinations of cationic lipids and cationic polymers together to form lipopolyplexes. ’In general, positively charged complexes of this type can be efficient at delivering genes to cells in culture but are not generally stable in the bloodstream or in tissues in vivo and thus are not active as in vivo gene delivery agents’, Hagstrom reports.
These are only a handful of the different approaches taken by groups and biotech companies around the world as they work to develop non-viral systems. But all these researchers agree that it will take time to develop the great potential of non-virals. And they do show great promise. Viruses are efficient at delivering genes into a variety of cell types but they have many well-documented drawbacks. The non-viral approaches, on the other hand, provide the ability to control all aspects of the components that go into the particle. ’This flexibility should ultimately allow for a simpler and less immunogenic delivery system’, believes Hagstrom.
Source: Chemistry in Britain
Acknowledgements
Maria Burke is a freelance science writer based in St Albans.
References
1. D. Oupicky et al, Mol. Ther., 2002, 5, 463.
2. T. H. Kowalczyk et al, Mol. Ther., 2002, 5, S435.
3. S. Hwang and M. E. Davis, Bioconjugate Chem., 2002, 13, 630.
4. M. E. Davis et al, Bioconjugate Chem., 1999, 10, 1068.
5. J. E. Hagstrom, Curr. Op. Mol. Therap., 2000, 2, 143.






No comments yet