A new class of molecular ‘photoswitch’ – stable in the solid state – can store solar energy upon excitation with visible light. This overcomes some critical challenges of conventional energy storage structures, such as thermal stability and optical sensitivity beyond the UV spectrum – both attractive properties towards scale-up and commercialisation.
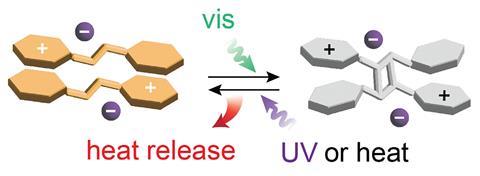
‘Our compounds store solar energy in highly strained chemical bonds,’ explains lead author Grace Han from Brandeis University in the US. ‘The absorption of light triggers a chemical change. An external stimulus sparks the reverse reaction, liberating the stored energy, usually as heat.’ These strained systems, stable for several decades, release the energy on demand upon thermal activation or UV irradiation. They can store up to 51J/g, which is enough energy to heat up 1g of water over 10 degrees.
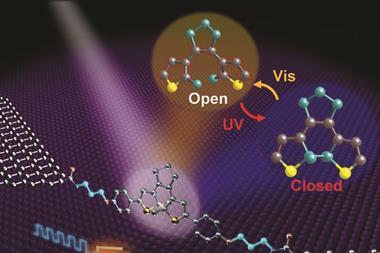
The photoactive compounds fit into the family of molecular solar thermal energy storage compounds, commonly called Most. The secret to this storage system is a styrylpyrylium motif, different to the usual Most structures – azobenzenes, hydrazones and fulvalenes. ‘It’s a new class of materials,’ comments Han. And it’s a double deal: ‘Our systems work in the solid state and absorb a wide wavelength range in the visible spectrum, key aspects for scalability and sustainability,’ she adds.
Traditionally, Most materials work best in solution and solid–liquid interphases, which requires big volumes for efficient setups and poses problems like leakage, pollution and fire hazards. Additionally, well-studied photoswitches absorb mostly UV light, which represents a rather narrow range of the solar spectrum. ‘We tuned the absorption to the visible range … and in the future, further functionalisation could convey even wider wavelengths,’ she adds.
‘The use of a larger part of the solar spectrum is very promising,’ says Kasper Moth-Poulsen, an expert in Most systems and smart materials, and an ICREA professor at ICMAB and UPC in Barcelona, Spain. ‘We need solid systems for applications in coatings and windows, for example.’
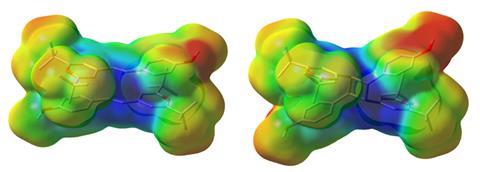
To work well in the solid state, researchers had to optimise ‘a lot of details in the crystal packing’, according to Moth-Poulsen. ‘The donor–acceptor nature of styrylpyrylium favours a particular packing pattern, which facilitates the cycloaddition reaction,’ explains Han. The [2+2] cycloaddition, triggered by sunlight, forms a strained cyclobutane that stores solar energy. ‘It’s hard to have the reaction in the solid state, the switch needs space,’ she adds. ‘Indeed, it’s quite remarkable, switching the systems deep inside a crystal is difficult,’ says Moth-Poulsen.
Historically, crystals had served as a synthetic strategy to prepare [2+2] cycloadducts with high regio- and stereoselectivity. ‘We really just rediscovered and redesigned photochemical crystal converting compounds,’ explains Han. The team studied styrylpyrylium structures with several substituents and different counter-anions, and assessed the stability and storage properties.
Moth-Poulsen values the ‘systematical exploration’ of different electron-donor and electron-withdrawing groups. ‘By tuning the properties, authors … not only tailor the optical properties, but also the [stability and] storage time,’ he adds. In this case, they demonstrated the solid-state storage-release process is repeated without decomposition or a decrease in efficiency for over 10 cycles, and increased the half-life for energy storage from four days to 32 years. This showcases the success of selecting the best substituents and counter-anions, which eventually enables efficient and long-term energy storage.
References
S Cho et al, Chem, 2023, 9, 1, (DOI: 10.1016/j.chempr.2023.06.007)






















No comments yet