A quick and simple way to create catalytic atom thick layers of platinum has been developed by US scientists
Atom thick catalytic layers of platinum can be deposited on surfaces from solution rapidly and cheaply thanks to a new technique developed by US scientists. The quality of the films produced is comparable to those produced by vapour atomic layer deposition, but uses a much simpler process.
Platinum films are used as catalysts in devices such as fuel cells, as well as in microelectronics and various other applications. Because of the rising price of platinum and the interesting properties of very thin films, it is desirable to make these films as thin as possible, explains Thomas Moffat from the National Institute of Standards and Technology in Gaithersburg, who led the project.
Atomically thin films of platinum can already be made using vapour deposition or molecular beam epitaxy, Moffat acknowledges, but these techniques involve expensive high vacuum chambers and each layer forms quite slowly as the added atoms skate around on the surface to find low energy sites. ‘We’re essentially using beaker chemistry and can lay down a monolayer in under a second,’ Moffat says, ‘so from an engineering perspective it’s much simpler.’
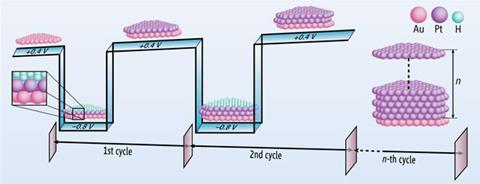
But to get a smooth, thin layer of platinum atoms on their surface, the team had to abandon traditional electrodeposition techniques. Normally, Moffat explains, deposition is done very slowly. The item is immersed in a bath containing a platinum salt and a very small potential is applied. The platinum complex is reduced and metal atoms are deposited first at steps or defects on the surface. ‘But platinum is happier growing on platinum than it is on gold,’ says Moffat, so you end up with islands that eventually join up into relatively thick, lumpy layers.
As part of their investigations, Moffat’s team tried cranking up the voltage. ‘We went right to the threshold of when you start reducing the protons in solution into hydrogen gas, which is not normally what you’d want to do,’ he says. Instead of getting a very thick film of metal laid down, they got a single atomic layer of platinum, capped with a layer of hydrogen atoms. Switching briefly to a positive potential oxidises the hydrogen atoms off the surface, leaving the platinum ready to add another layer if needed.
This, says Jay Switzer from Missouri University of Science and Technology in Rolla, US, is the biggest advantage of the technique. ‘In one beaker, just by pulsing the potential back and forth, you can put down one monolayer at a time. So if you wanted to look at properties of a material as a function of thickness, you can grow anywhere between one and several layers fairly easily.’ This kind of investigation will be particularly useful for people working on catalysts and magnetic materials, where the properties of thin film materials can vary considerably.






2 readers' comments