A compound which features multiple bonds between lead and carbon has been isolated and characterised. This heteronuclear ‘plumbyne’ fills a gap among group 14 alkyne analogues and could offer a starting point to create rarer main group species.
Bonding in organo-lead compounds is typically limited to single lead–carbon bonds, owing to lead’s large atomic size and poor orbital energy match to other atoms. Relativistic effects also suppress lead’s ability to form higher-order π bonds. Compounds where lead forms multiple bonds to other lead, transition metal or non-metal atoms exist, but are rare.
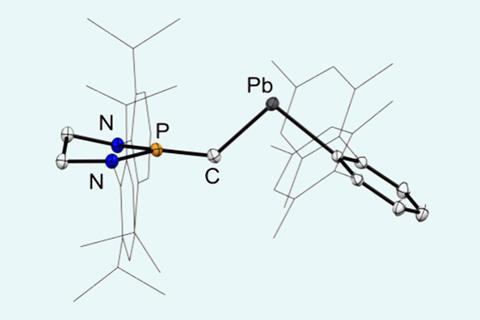
By balancing electronic and steric factors, researchers in China have now created a compound that features multiple bonds between lead and carbon.1 An aryl group with bulky substituents and an N-heterocyclic carbene containing phosphorus are connected to the ends of a central lead–carbon bond. The team previously used a similar structure to create the lighter tin ‘stannyne’ equivalent.2
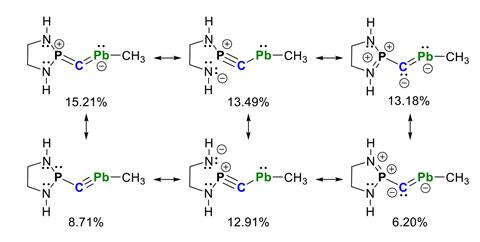
X-ray diffraction showed that the lead–based compound adopted a trans bonding arrangement, with a central carbon–lead bond length of 2.172Å. The substituents were shown to be nearly orthogonal to each other, which is thought to improve orbital overlap and increase stability of the P–C–Pb linkage. Computational analysis of a simplified structure revealed that the most prevalent resonance form features a P=C=Pb bonding pattern.
Reactions using the compound were possible, where addition of ammonium hydrochloride easily saturated the multiple carbon–lead bond, which the researchers attribute to the bond’s relatively weak nature. Using a tin-based reagent saw bond–metathesis occur, creating a new tin compound and lead byproduct, though the exact reaction mechanism remains unclear. The researchers hope that this plumbyne compound will act as platform to access other rare main group species.
References
1 X-F Wang et al, J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c17050
2 X-F Wang et al, Nat. Chem., 2025, 16, 1673 (DOI: 10.1038/s41557-024-01555-4)


















No comments yet