A new pyrolysis protocol for chemical recycling of polymers has been developed by researchers in the US. The technique, which uses pulses of electricity to heat the waste polymer, minimises side reactions and thereby produces more monomer.
As the inadequacies of traditional recycling to handle plastic waste become ever more apparent, scientists turn increasingly to chemical methods, either using it as a feedstock for producing other materials or depolymerising it completely so it can be made into pristine polymer from scratch. Many embryonic depolymerisation schemes, however, require replacing today’s commercial polymers with chemical alternatives that can be depolymerised using specific reactions.
At present, one widely used method of chemical recycling for many plastics is pyrolysis, or thermal decomposition. However, the equilibrium yield of the monomer is often very low. In the absence of a catalyst, for example, pyrolysis of polypropylene produces only about 10% propylene as a variety of competing reactions produce undesirable and sometimes toxic side products such as paraffins and aromatics. Even with optimised catalysts, the yield is often below 25%.

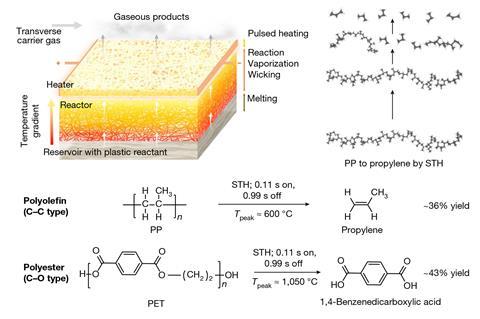
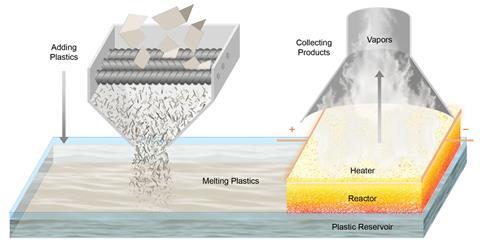
Researchers led by Yiguang Ju of Princeton University in New Jersey and Liangbing Hu at the University of Maryland, College Park advanced a technique they developed last year in which short pulses from an electrical heater periodically raise the temperature of the reaction mixture and allow it to cool.1 This prevents the reaction from reaching thermodynamic equilibrium and allows optimisation of the product yield. For the new work, they designed a reactor comprising a porous carbon felt bilayer with the pulsed heating supplied at the top.
Polymer reactant melts enter the lower layer of the reactor and are drawn up through the porous material and gradually decompose as the temperature increases. As the molecules become smaller, their volatility increases. They are therefore expelled at the top as gases, drawing more liquid polymer in at the bottom. As the temperature oscillates, the simpler depolymerisation reactions are favoured over side reactions that require additional thermalisation and have more complex mechanisms. Moreover, the spatial gradient, in which smaller, partially-depolymerised molecules reach higher temperatures, favours depolymerisation. The energy requirements – which are lower because of the pulsed scheme – could potentially be supplied efficiently by renewable electricity.
The overall monomer yield was about 36% from depolymerisation of polypropylene with no need for a catalyst. ‘The other 64% will be composed of small molecules such as methane, acetylene and larger molecules – there will be some aromatics – and we are now working on further improving the efficiency,’ says materials chemist Qi Dong from the University of Maryland.
Anthony Ryan of the University of Sheffield in the UK is not enthusiastic, however. ‘This paper is typical of research that is well intentioned but not really well thought out,’ he says. When the carbon emissions associated with pyrolysis are considered, he argues that the carbon footprint for fuel production from pyrolysis is so much larger than the production of virgin fuels that, even if half the residue was suitable only for combustion, a life-cycle analysis would show the process to be more carbon-intensive than virgin-fuel production. He believes the process might have potential if it can synthesise monomers that are otherwise difficult to recover, but concludes that, given the 100 million tonnes of polyolefin production, the abstract’s claim that it ‘potentially offers a solution to the global plastic waste problem’ is a stretch.
‘Pyrolysis-based plastic recycling approaches are often energy intensive and carbon-heavy, indeed,’ Hu responds. ‘However, instead of conventional fossil-fuel-burning based heating, our process is powered by electrified heating, which can be coupled with the increasingly available renewable electricity to significantly reduce the carbon footprint. In addition, the electrified heating is pulsed, which can be used to improve the energy efficiency.’
References
1 Q Dong et al, Nature, 2022, 605, 470 (DOI: 10.1038/s41586-022-04568-6)
2 Q Dong et al, Nature, 2023, 616, 488 (DOI: 10.1038/s41586-023-05845-8)

















No comments yet