Researchers have developed a highly selective technique for synthesising nitrogen-containing heterocycles by inserting nitrogen atoms into arenols. The technique adds to the growing field of skeletal editing and brings the benefit of being both selective and a one-pot process.
While most organic synthesis techniques involve changing atoms or groups on the periphery of a molecule, skeletal editing allows researchers to alter a molecule’s underlying core. By making such changes directly, chemists can access novel heterocycles, and develop cheaper and more efficient ways to synthesise pharmaceutically important compounds. Sudipta Raha Roy, an organic chemist at the Indian Institute of Technology Delhi who wasn’t involved in the research, states that although skeletal editing is still in its infancy, it is changing at a fast pace.
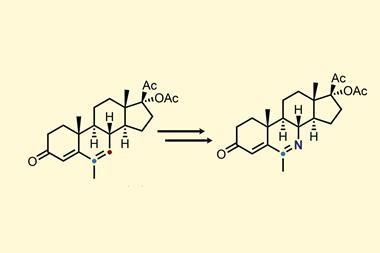
Skeletal editing typically involves inserting, removing or swapping a carbon, oxygen or nitrogen atom. Of these, inserting nitrogen into an aromatic ring is particularly challenging because it is difficult to break the aromaticity and high bond dissociation energy of an aromatic carbon–carbon bond. Hao Wei and colleagues from Northwest University, China, overcame this problem by using arenols as their substrates. This also allowed them to control where they inserted the nitrogen. ‘Regioselective control has always been difficult. The use of phenol substrates also provides a way to solve the regioselectivity problem,’ says Wei.
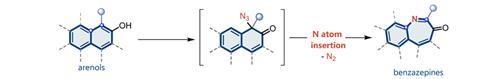
Vignesh Palani, a synthetic organic chemist at the Indian Institute of Science Bangalore, says another strength of the work is that nitrogen can be inserted in one step. Where previously a nitrogen-containing group, in this case an azide, needed to be pre-installed on the compound, ‘they do everything in a one pot reaction’. Another advantage, Palani points out, is that some previous nitrogen insertions required photolysis, whereas this reaction does not so it may be easier to scale up.
So does the technique have any limitations? Wei identifies just one; it needs an electron-withdrawing group or aromatic group at the ortho position. ‘We have spent almost a year trying to solve this problem, but there is no progress at this stage.’
Wei envisages that his team’s technique will be used not only to synthesise novel organic compounds, but also in the synthesis and optimisation of new organic materials. As well as expanding the scope of aromatic substrates, Wei hopes to adapt the reaction beyond nitrogen atoms ‘to include the insertion of boron, silicon, phosphorus and other atoms’.











No comments yet