A rubber-like material can mend itself when cut or broken
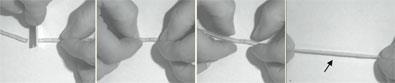
Chemists in France have made a new, rubber-like material that can be repaired simply by pressing cut or broken pieces together at room temperature.
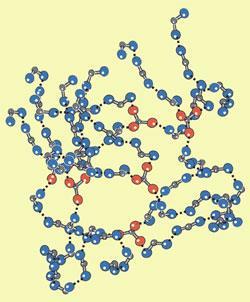
Ludwik Leibler’s group at the Soft Matter and Chemistry Laboratory, a joint unit of CNRS and ESPCI (Ecole Sup?rieure de Physique et de Chimie Industrielle) at Paris, set out to create materials that, like rubber, contain networks of long molecular chains. But instead of the covalent bonds that hold rubber together, they wanted to find molecules that would link together and cross-link with each other through hydrogen bonds. The group chose small molecules (carboxylic acids and amines) that had either two or several connection points.
Their first attempt led to a glassy plastic that displayed rubber-like properties at 90?C. In order to soften the material at room temperature, they added small amounts of solvent, and obtained a new material that turned out to be just as elastic as ordinary rubber. This low-molecular rubber has the extra benefit that cuts and breaks can be repaired by simply pressing the cleavage sites together because the hydrogen bond network holding it together reconnects within minutes. This property also makes the material easy to recycle.
’The beautiful work brings the fields of self-healing materials and supramolecular polymers together; a long-held wish is now reality,’ Bert Meijer, a professor of molecular sciences at the Eindhoven University of Technology in the Netherlands, told Chemistry World. ’The new work will give an additional boost to the many options of supramolecular materials and they will soon reach the market place, especially since many systems are very easy to synthesize and are cheap.’
Michael Gross
References
et alNature451, 977 (DOI: 10.1038/nature06669)






No comments yet