Cobalt polysulfide shows promise as low-cost alternative to platinum group catalysts
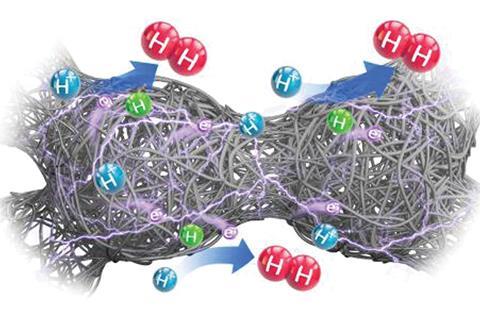
Cobalt polysulfide shaped like a silk cocoon exhibits extremely high catalytic activity for the hydrogen evolution reaction, new research shows.
The hydrogen evolution reaction is a sustainable and clean way to generate hydrogen from water, but the rarity and high cost of the platinum group metals that are typically used to catalyse the reaction limit its applicability.
The large exposed sulfur sites and bridging S22− sites in transition metal polysulfides suggest they would make effective hydrogen evolution catalysts. However, they are difficult to prepare.
Now, an international team of researchers has shown they can prepare a cobalt polysulfide-based material that shows hydrogen evolution activity comparable to commercial platinum on carbon catalysts using a simple one-step hydrothermal method. The catalyst consists of nanofibers interwoven into hollow spheres, forming a three-dimensional conductive silk cocoon-like structure. The researchers attribute the material’s catalytic activity to its unique interconnected structure and polysulfide composition.
The team believes their work will aid the design of future hydrogen evolution electrocatalysts and that their material could potentially be used in other technologies such as batteries and supercapacitors.
References
This article is free to access until 12 September 2018
C Wang et al, Energy Environ. Sci., 2018, DOI: 10.1039/c8ee00948a










No comments yet