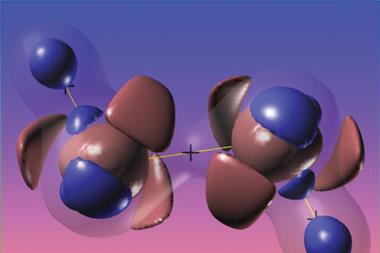
Lewis structures are the universal language chemists around the world use to represent molecules. However, some chemists think that these conventions can be misleading. Gerard Parkin from Columbia University in New York, US, is one of those chemists and he has come up with a new approach to represent these structure that he says makes them much clearer. This solution is particularly helpful for representing electron-deficient compounds such as diborane.
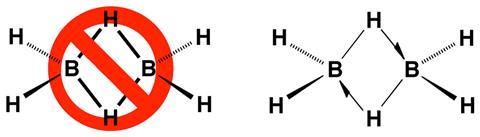
The representation of chemical bonds as lines for normal covalent bonds or arrows for dative covalent bonds is very straightforward. But three-centre–two-electron structures cause some additional problems: if bonds are represented with lines, they may be mistaken for conventional covalent liaisons, while if they are depicted with dotted connections, they may be confused with intermolecular interactions. Parkin’s solution is a half arrow (⇀).
‘The purpose of using a half-arrow (rather than a full-arrow) for the bridging hydrogen atom is to emphasise that the arrow does not correspond to donation of a lone pair but rather donation of a bond pair,’ he explains in the paper. Representing electron-deficient bonds in this way, it would be easier for chemists to count electrons around a particular atom, which is particularly useful in organometallic chemistry. As the half arrow represents the donation of a whole pair, the electron count at the tip would always be two.
This novel concept could find uses beyond representing compounds with hydrogen bridging atoms like diborane, also clarifying many organometallic structures, and even allowing chemists to infer whether additional metal–metal bonds exist. Moreover, it could simplify the depiction of agostic compounds – a class of compounds that exhibit interactions between a C−H bond and a metal centre. Agostic compounds are reaction intermediates in many catalytic transformations, and key to important chemical processes such as C–H activation, metathesis and polymerisation.
Parkin believes that, together with the existing conventions, this new approach will afford ‘a simpler way to evaluate the chemical reasonableness of a molecule’.
References
G Parkin, J. Chem. Ed., 2019, DOI: 10.1021/acs.jchemed.9b00750












No comments yet