Synthetic production of the naturally occurring anti-cancer medicine paclitaxel may be one step closer with the discovery of the enzymes responsible for producing baccatin III, a complex precursor in the biosynthesis of the drug. Previous methods for synthesising paclitaxel in the lab take up to 30 steps, the use of hazardous reagents and sourcing of baccatin III from yew trees or plant cell culture. Now a team of researchers in China report just nine genes are needed to produce baccatin III in tobacco plants.
Paclitaxel, sold under the brand name Taxol, is used to treat several types of cancers and was discovered in the Pacific yew in the 1960s. Synthesis of baccatin III with a view to producing the drug in the lab has frustrated researchers for decades, however. According to co-author Xiaoguang Lei, a chemist at the Beijing National Laboratory for Molecular Sciences, baccatin III biosynthesis is complicated because each enzyme can produce multiple substrates. By expressing a sub-family of cytochrome P450 genes, called CYP725A, in cell cultures and feeding those cells known substrates the team could untangle which genes were involved in each step.
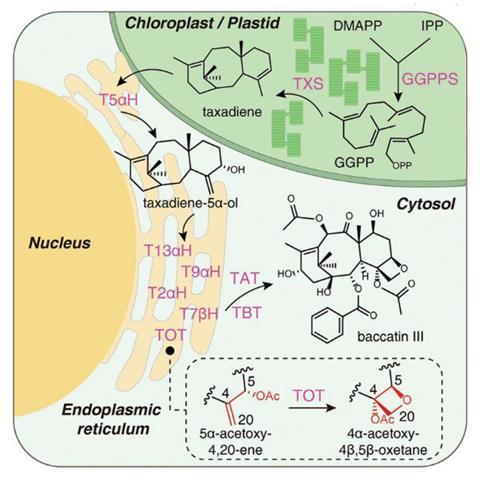
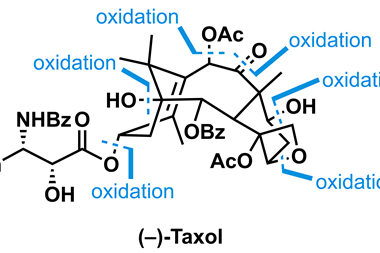
According to Lei, the crucial missing steps for baccatin III synthesis were the enzymes responsible for oxetane ring formation and C9 oxidation. A new bifunctional enzyme the team named taxane oxetanase (TOT) was responsible for the former. ‘TOT is a bifunctional oxygenase that directly converts the alkene moiety into the epoxide and the oxetane ring,’ said Lei. The formation of the epoxide ring was previously thought to be an essential intermediate for the oxetane ring, but this discovery revises that hypothesis. The team then identified an enzyme called T9αH which performed the C9 oxidation. In total, they identified nine genes and their enzymes required to produce baccatin III – far fewer than previous estimates of up to 14. To confirm the pathway, they expressed the genes in modified tobacco plants, producing baccatin III.
‘For now, this is not efficient enough for production purposes though, as shown in the paper, but if one day it becomes viable, it could be a new way to produce the precursor for paclitaxel,’ says Aurélien de la Torre, a chemist at the Orsay Institute of Molecular Chemistry and Materials, France.
Lei’s team is continuing to work on the metabolic pathway and hopes to develop ‘a synthetic biology approach to produce baccatin III in large scale’. The new multifunctional enzymes may also aid production of other oxetane-containing Taxol analogues.
References
B Jiang et al, Science, 2024, DOI: 10.1126/science.adj3484










No comments yet