I will admit that there are years when I feel a more personal connection with the chemistry Nobel than others. That’s not the common complaint that many of the recent awards have been pointed more in the biology direction. I’ve tried never to have a gatekeeper mentality about chemistry. You can end up being so protective of some Zone of Purity that you don’t realise just how small it’s become and how many interesting and useful things there are just outside it.
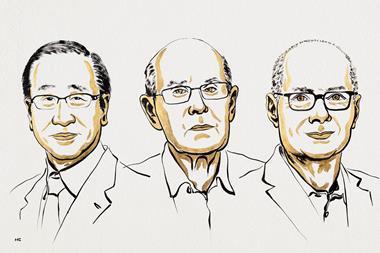
But I am an organic chemist at heart, and I’ve also managed to keep a connection to bench work during my career. So I was happy to see the 2025 award to Susumu Kitagawa, Richard Robson, and Omar Yaghi for metal-organic frameworks (MOFs). Even the most relentless intellectual sentries have to admit that this was truly chemistry. What’s more, it partakes of inorganic, organic, physical, and analytical branches all at the same time. But what made me smile was knowing that I had actually gone into the lab and made some MOFs with my own hands, and collected x-ray data on many of them, too.
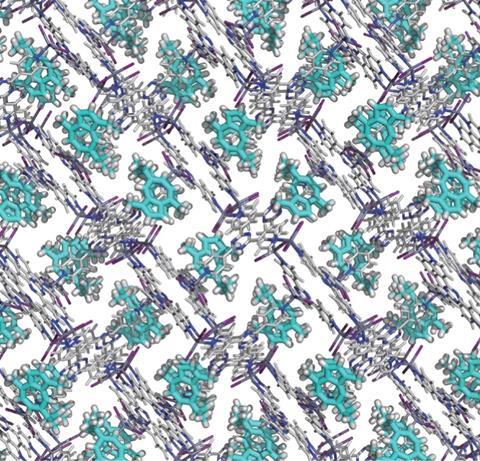
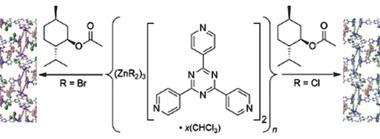
That was for a special project a few years ago, when the company I was working for called for unusual drug discovery ideas that didn’t fit into any of the existing categories. I am always ready for someone to ask me a question like that, and I immediately thought of some work that had been recently published by Makoto Fujita and his group at the University of Tokyo, Japan. The MOF field had been (and still is!) putting a lot of work into the idea of guest molecules inside the chambers and channels of these odd crystalline materials. What Fujita’s team found was that some MOFs could act as unexpectedly orderly storage sites for such molecular guests, in a sort of ‘one per room’ hotel plan. If you soaked these into the MOF and then collected x-ray data, you got both the structure of the MOF framework but also data on the guest molecules. These were now such a regular repeating part of the crystalline framework that they produced diffraction data of their own, allowing their structures to be solved as well.
The parade of yellow, green, purple, red, and colourless crystals in all sorts of forms was dazzling
The exciting thing about this was that it could be applied on a very small scale, and to compounds that were difficult or impossible to crystallise by themselves under ambient conditions. My thoughts immediately turned to metabolites in drug discovery: enzyme-altered versions of our drug candidates produced in the liver and other tissues, with difficult-to-predict structures and stereochemistry – and usually available only in very small amounts. Why not use MOF crystals to nail all those questions down in one pass? So I set about trying to see how general the phenomenon was and how it could be applied.
And let me tell you, making the MOFs themselves was quite possibly the most fun I have ever had in the lab. You combine metal salts and the multi-pronged organic ligands in some high-boiling solvent and heat them up for a day or two, and if you’re lucky you start growing vivid, sparkling crystals on the sides of the tubes. Just seeing things crystallise from such strong solvents at high temperatures was odd enough – normally such conditions would dissolve almost anything. But the parade of yellow, green, purple, red, and colourless crystals in all sorts of forms was dazzling. Then you’d soak out the original solvents and soak in solutions of test drug substances, and it was off to the x-ray lab (or the synchrotron!) to see what you had.
Making new things is a fundamental joy of bench chemistry, and I have never experienced such a concentrated version of it as this
What I found was that you could indeed get some of these things to order themselves inside some of the MOF crystals, but we could find no useful general systems. It was a procession of sporadic one-off results, and not even all of the successes were successful enough. The problem needed much more work and resources than I could give it, unfortunately. But Fujita’s lab and others have continued over the years and have made real progress, and I still have hopes for the general idea.
I was disappointed in that result, but I still found the work very satisfying and compelling. Making new things is a fundamental joy of bench chemistry, and I have never experienced such a concentrated version of it as this. What chemist doesn’t enjoy crystals in all their forms? No, I would be glad to fail again like this: aiming at a high goal and having a tremendous time doing it. Scientia experientia est, and over 40 years in the lab I don’t think I’ve ever felt more like a scientist than I did then.














No comments yet