Harnessing new interactions opens doors for molecular control
A new field is always exciting. Even more so when it’s based on an apparently ‘new’ non-covalent interaction. Anion-π interactions have long been mooted – albeit somewhat controversially – but in recent years, experimental evidence for their function has built to the stage where we can be reasonably confident they are real.1
In contrast to widely accepted cation-π interactions, whereby electron-rich aromatic systems stabilise cations, stabilisation of anions by electron-deficient (π-acidic) aromatic surfaces is a rarely exploited phenomenon. Beyond the work of Stefan Matile and his team at the University of Geneva, Switzerland, rational applications in catalysis is hitherto unknown.
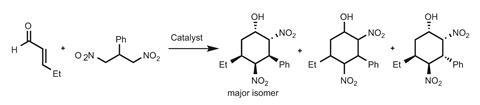
The team’s early work focused on exploiting these ‘exotic’ interactions in complex functional materials that use light to generate chemical or electrical energy. More recently they have begun probing applications in anion-π catalysis. In 2013, they showed that highly electron-deficient aromatic surfaces can stabilise the anionic transition state of the Kemp elimination2 – a remarkably useless synthetic reaction, but fundamental in studying organic mechanisms. The team has now shown that π-acidic aromatic systems can effectively catalyse asymmetric iminium-nitroaldol cascade reactions, forming cyclohexanes with five contiguous chiral centres (figure 1).3
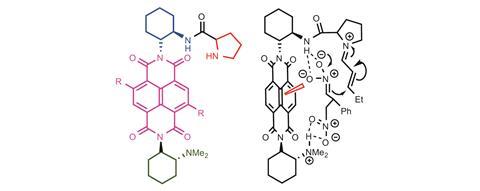
The team prepared a multifunctional napthalenediimide (NDI)-derived catalyst, consisting of an organocatalytic proline component to activate the enal for conjugate addition; a trans-cyclohexyldiamine as a rigid scaffold ensuring substrates are held close to the π-acidic surface of the NDI; the nitronate-stabilising NDI π-acidic surface itself; and a tertiary amine base that is critical to reactivity (figure 2).
Using this initial catalyst, the team can form cyclohexane products as mixtures of diasterioisomers. Using nitrobenzene as the solvent gives the best results: 90% yield, a diastereomeric ratio of 63:19:18, and an enantiomeric excess of 58% for the major diastereoisomer. The team suggests nitrobenzene’s electron deficient nature (compared to other aromatic solvents such as toluene and benzene) enhances the π-acidity of the NDI surface through π-stacking interactions.
Modifying the substituents on the periphery of the NDI, the researchers generated a series of catalysts with increasing π-acidity, which directly correlates with the rates of reaction and stereoselectivity. This strongly suggests that the anion-π interaction plays a part in the catalysis. Increasing the steric bulk at the periphery further improved selectivity, giving enantiomeric excesses of up to 70%.
Using nitrobenzene as the solvent, the team compared this system with results using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a base, along with a proline-derived organocatalyst developed by Karl Anker Jørgensen of Aarhus University in Denmark, and Yujiro Hayashi of Tokyo University of Science and Technology, Japan. Although the π-acidic NDI comes out on top under these conditions, the classical system does give higher stereoselectivity in CH2Cl2. That said, one of the Matile group’s catalysts does achieve the best diastereomeric ratio reported to date (69:12:19).
It is unlikely that these systems are going to find many synthetically useful applications anytime soon, but who knows what the future holds. It is not a huge leap to imagine the stabilisation of anionic tetrahedral intermediates in many common reactions, but it could get really interesting if modulating the reactivity of metals or reaction intermediates in metal-catalysed reactions held in the proximity of these surfaces is possible. Whatever the progress in catalysis, the increased understanding and modulation of these fascinating anion-π interactions will contribute towards Matile’s ultimate goal of building of increasingly complex and functional multicomponent systems.
References
1 R E Dawson et al, Nat. Chem., 2010, 2, 533 (DOI: 10.1038/nchem.657)
2 Y Zhao et al, Angew. Chem., Int. Ed., 2013, 52, 9940 (DOI: 10.1002/anie.201305356)
3 L Liu et al, J. Am. Chem. Soc., 2016, 138, 7876 (DOI: 10.1021/jacs.6b04936)












No comments yet